Resources & Support
Protocols - Antigen Affinity Purification of Antibodies
Cytokines are signaling proteins necessary for cell-to-cell communication throughout the body. In accordance with their role in preserving vital biological functions, the amino acid sequence of homologous cytokines from different mammalian species is generally highly conserved, rendering them poor immunogens for antibody production.
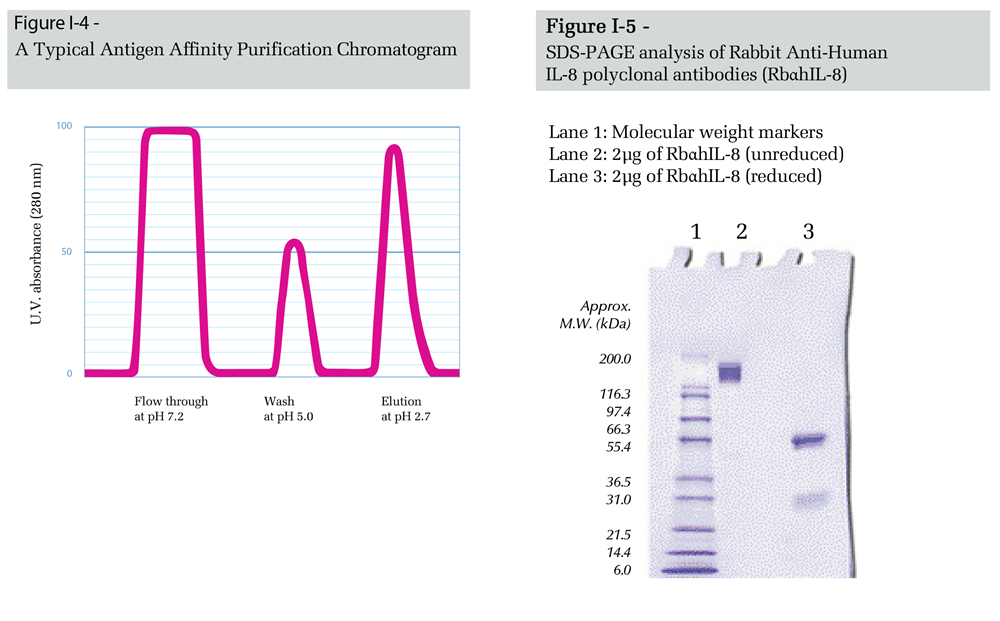
Consequently, the sera obtained from host animals after immunization with cytokines (Figure I-1) typically contain minute amounts of cytokine-specific antibodies. Isolation of these antibodies by standard purification procedures (e.g. ion-exchange chromatography) is tedious and ineffective. On the other hand, purification protocols which exploit the binding affinity of antibodies to certain biomolecules have yielded much better results.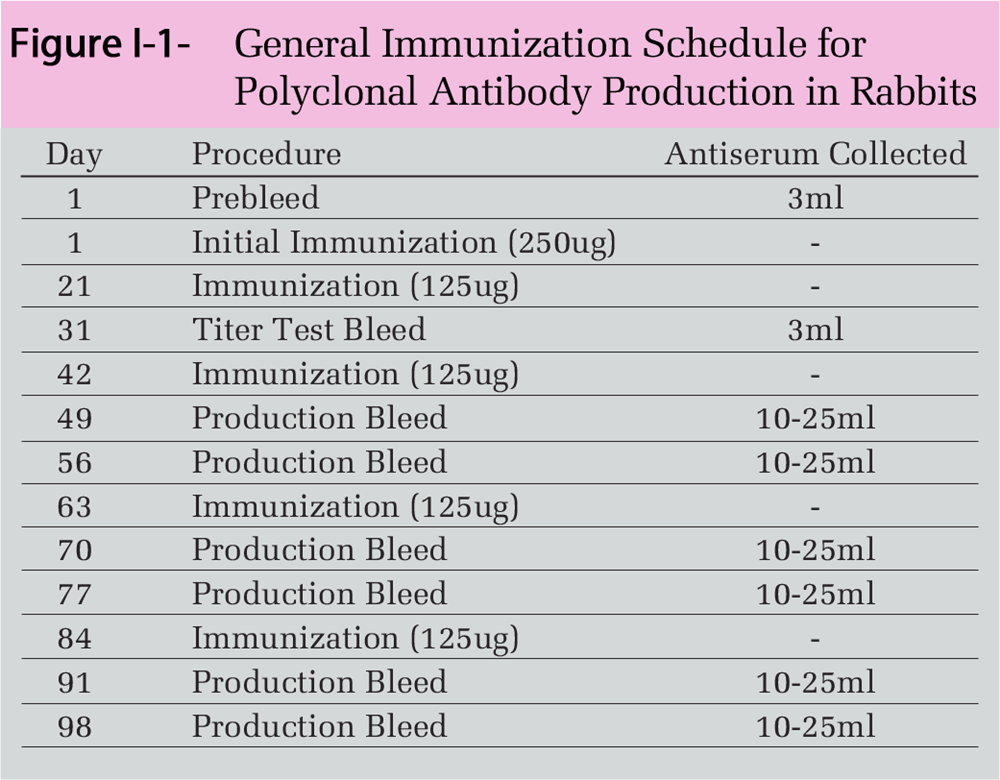
For example, Protein A and Protein G are bacterial (Staphylococcus)-derived proteins that possess high binding affinity toward the Fc (Fragment crystallizable) region of immunoglobulins (IgGs), and when attached to a solid-support matrix, enable efficient separation of IgGs from other serum constituents. Protein A/G affinity purification of serum-IgGs typically enriches the desired antibody by more than 100-fold. Yet, in the case of anti-cytokine antibodies, the total IgG fraction often contains less than 0.2% of the desired antibody.
The large quantity of unrelated IgGs found in these preparations not only interferes with quantification of the relative amount of the cytokine-specific antibody, but also considerably increases the background noise when the antibody is used in analytical procedures such as ELISA, neutralization, immunohistochemistry, and Western blotting. A superior method for isolating specific polyclonal antibodies from antiserum is by affinity chromatography, which exploits the specificity of antibody-antigen interactions (Figure I-2).
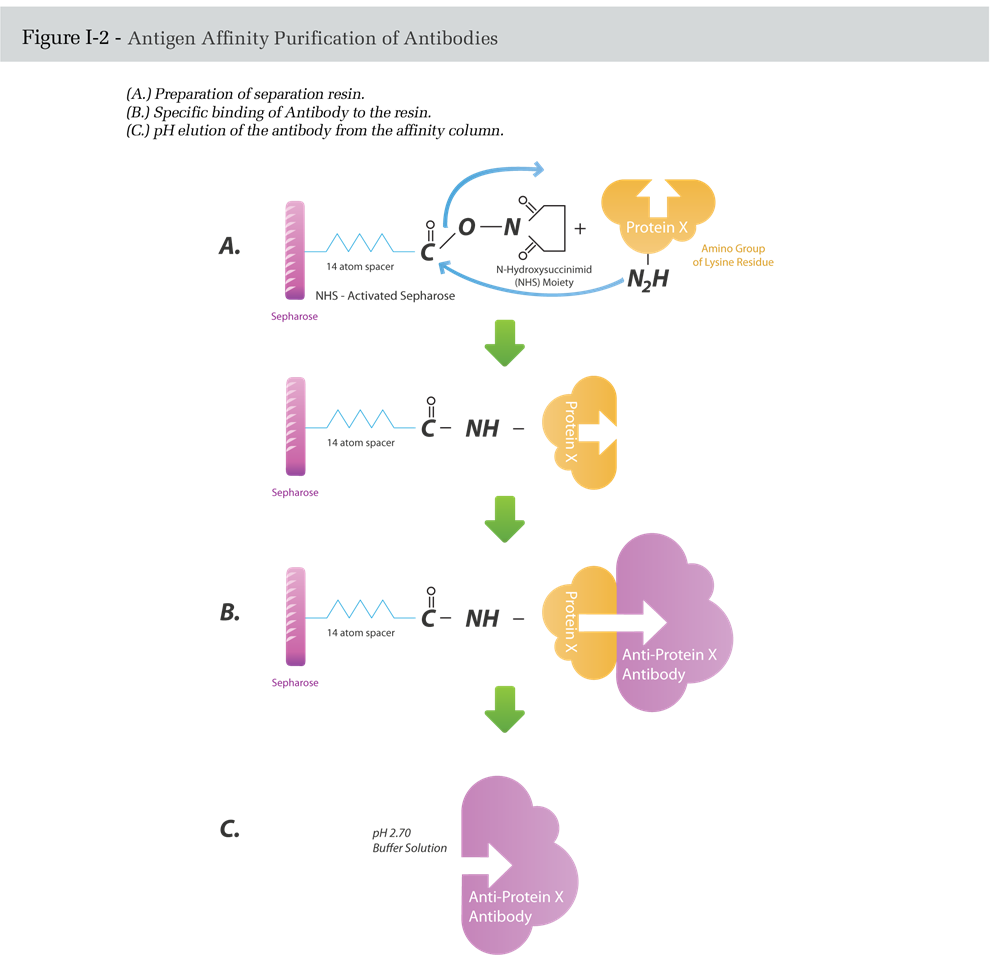
Here, the separation medium consists of a solid-support resin to which the antigen is attached through stable covalent bonds. The immobilized antigen is then used in a column chromatography setup to selectively capture antigen-specific antibodies, while other serum proteins and unrelated immunoglobulins are washed away (Figure I-3).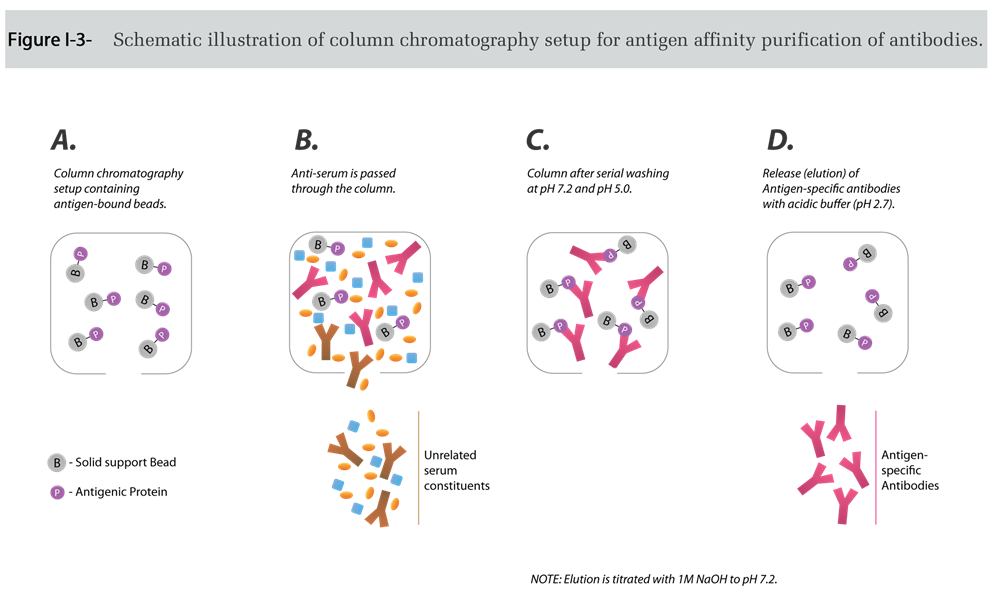
The antigen-bound antibodies can be eluted from the column by an acidic solution (Figure I-4), which is promptly brought back to physiological pH to prevent acid-catalyzed antibody denaturation. This method typically yields >95% pure specific antibodies (Figure I-5).