What Are Immune Checkpoints?
Facilitate Your Immune Regulations Study
The recognition of the antigenic peptide-MHC I/II complex on antigen presenting cells (APCs) through the T cell receptor (TCR) initiates T cell-mediated immunity. However, the interaction of antigen-MHC complexes with the TCR is not sufficient for activation of naïve T cells. Additional costimulatory signals are required for T cells to become fully activated. Once activated, T cells proliferate and migrate to sites of inflammation where they attack cells expressing relevant antigens, and destroy them either directly or indirectly through other effector cells or factors (e.g. cytokines). Effective immune response is imperative to defend against invading pathogens as well as against malignant neoplasms; however, dysregulated immune response can result in chronic inflammation, which leads to tissue damage and autoimmunity.
Immune checkpoints are inhibitory regulators of the immune system that are crucial to maintaining self- tolerance, preventing autoimmunity, and controlling the duration and extent of immune responses in order to minimize collateral tissue damage. These immune checkpoints are often overexpressed on tumor cells or on non-transformed cells within the tumor microenvironment, and compromise the ability of the immune system to mount an effective anti-tumor response.
Immune responses are regulated by a balance between co-stimulatory and inhibitory signaling pathways, which are stimulated by an assortment of receptors and their respective ligands (Figure 1). The ability to shift the balance towards a desired response can offer a way to ameliorate a variety of diseases.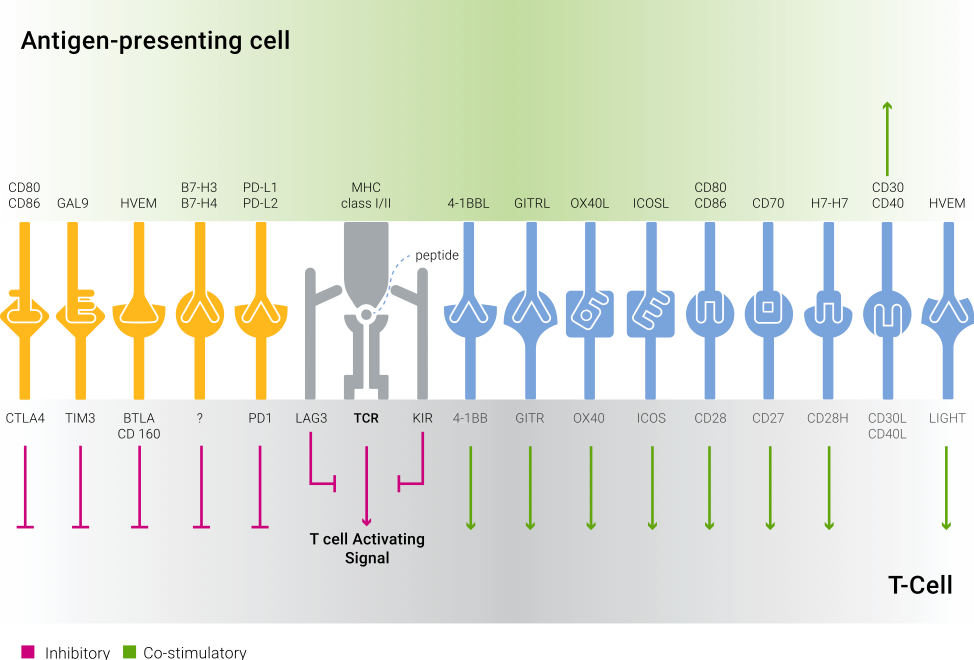
| Figure 1: Co-signaling interactions in T cells. Co-stimulatory molecules deliver positive signals to T cells following their binding to ligands and receptors on APCs. Inhibitory (Checkpoint) molecules deliver negative signals to T cells upon interaction with their counterparts on the APCs. |
The blockade of immune checkpoints is among the current most promising approaches for activating therapeutic antitumor immunity and circumventing the immune resistance exhibited by many tumors. The first immune checkpoint therapy approved by the FDA targeted the Cytotoxic T‑lymphocyte associated antigen 4 (CTLA4) pathway (Ipilimumab, trade name Yervoy, Bristol-Myers Squibb). Subsequently, the FDA approved two treatments targeting the programmed cell death protein 1 (PD-1) pathway (Pembrolizumab, trade name Keytruda, Merck; and Nivolumab, trade name Opdivo, Bristol-Myers Squibb). [4]
Improved understanding of immunomodulatory pathways spurred the study of numerous immune targets as potential anti-cancer therapies, including studies that investigate the synergistic effect of combining such therapies.
The robust anti-tumor responses and tumor regression that are achieved by targeting immune checkpoint pathways suggests that the opposite strategy, of engaging these molecules, may alleviate inflammation in autoimmune diseases. This approach is supported by the FDA approval of the humanized fusion protein CD152-IgG1 (Abatacept, trade name Orencia, Bristol-Myers Squibb). Abatacept inhibits T cell activation by selectively blocking the co-stimulatory signal induced by interaction of CD80/CD86 ligands with CD28, which in turn inhibits T cell proliferation and B cell immunological response.
In the future, the examination of immune infiltration and expression patterns of immunoinhibitory markers will be used to optimize treatment to combine both checkpoint blockers and immune agonists.
The B7-CD28 Superfamily
The B7 family of ligands and the CD28 family of receptors belong to the Immunoglobulin SuperFamily (IgSF). Interactions between B7 ligands and CD28 receptors play an essential role in regulating T cell response by eliciting both positive co-stimulatory and negative inhibitory signals. There are currently ten known members of the B7 family: B7-1 (CD80), B7-2 (CD86), B7-H2 (ICOSL), programmed death ligand 1 (PD-L1), programmed death ligand 2 (PD-L2), B7-H3, B7-H4, B7-H5, B7-H6, and B7-H7; and five known members of the CD28 family: CD28, CTLA-4, ICOS, PD-1, and B- and T-Lymphocyte Attenuator (BTLA).
CD28 and B7-1/B7-2
CD28 is the only B7 receptor that is constitutively expressed on naïve T cells and provides co-stimulatory signals, which are required for T cell activation and survival. Interaction of CD28 and its ligands, B7-1 (CD80) and B7-2 (CD86), is involved in T cell activation, stimulation of cell proliferation and cytokine production, and promotion of T-cell survival. B7-1 expression is upregulated in APCs when activated by Toll-like receptor ligands, while B7-2 is constitutively expressed on APCs.
ICOS and ICOSL
ICOS is expressed on activated T cells, while its ligand, ICOSL, is expressed on APCs such as B cells, macrophages, monocytes, and dendritic cells (DCs). The interaction of ICOS with ICOSL mediates T cell activation and expansion, is involved in T cell dependent B cell activation, and Th cell differentiation.
CTLA-4 and B7-1/B7-2
CTLA-4 expression is upregulated rapidly following T cell activation and CD28 ligation on the surface of T cells. It is also expressed on regulatory T cells (Tregs) as well as on some types of non-T cells, both normal and malignant. CTLA-4 and CD28 share the same B7 family ligands, B7-1 and B7-2, which are both expressed on APCs; however, CTLA-4 has a 20-100 fold higher affinity than CD28. Ligation inhibits cell proliferation, cytokine production and cell cycle progression. CTLA-4 competes with CD28 for B7-1/B7-2 ligands, thereby blocking the CD28 co-stimulatory signal that is necessary for robust T cell activation and effector function. An immunotherapy treatment based on blocking the inhibitory effect of CTLA-4 has been approved by the FDA.
PD1 and PD-L1/PD-L2
PD-1 is primarily expressed on activated T cells, natural killer (NK) cells, B cells and certain myeloid cells. When activated, PD-1 signals to negatively regulate the immune response. There are two known ligands of PD-1: PD-L1 and PD-L2. PD-L1 is expressed on numerous immune cell types, including T cells, B cells, DCs, and on many tumor cells; while PD-L2 expression is induced on DCs and macrophages by IL-4, LPS and INF-γ. [6] PD-1 plays an important role in reducing autoimmunity and promoting self-tolerance by preventing the activation of T-cells and down regulating immune responses. PD-1 induced inhibition is achieved through promoting apoptosis in antigen specific T cells in lymph nodes, while simultaneously reducing apoptosis in Tregs. Research has shown that tumors exploit PD-1 signaling to evade immune detection, the importance of which is highlighted by the FDA’s approval of immunotherapy treatments using PD-1 signaling blockers to induce antitumor response.
CD28H and B7-H7
CD28H (CD28 homolog) is widely expressed, mainly by epithelial and endothelial cells. It is also expressed in lymphoid organs and in peripheral blood mononuclear cells, such as NK cells and CD3+ T cells. Its ligand, B7-H7, is highly expressed in B-cells, dendritic cells, monocytes and macrophages, but not in T cells. The interaction of CD28H with B7-H7 selectively co-stimulates human T cell growth and cytokine production in the context of TCR-mediated activation.
TIM-3 and GAL9
TIM-3 (T cell Immunoglobulin Domain and Mucin Domain 3), which belongs to the IgSF, is highly expressed on Th1 lymphocytes and CD11b+ macrophages and is upregulated on activated T and myeloid cells. TIM-3 regulates macrophage, activation and inhibits Th1 mediated immune responses to promote immunological tolerance. One of its ligands is GAL9 (Galectin-9), which belongs to the galectin family of lectins. The binding of GAL9 to TIM-3 can negatively regulate Th1 immune response, enhance immune tolerance and inhibit antitumor immunity. Dysregulation of the TIM-3/GAL9 pathway is implicated in many chronic autoimmune diseases, such as multiple sclerosis and systemic lupus erythematosus.
KIR and MHC-I
KIRs (Killer cell immunoglobulin-like receptors), a family of receptors belonging to the IgSF, are expressed on NKs and subset of T cells. KIRs regulate the killing function of these cells by interacting with MHC-l molecules, which are expressed on all cells types. Most KIRs inhibit the cytotoxic activity of NKs upon interaction.
B7-H3
B7-H3 (B7 homolog 3) from the B7 family of the IgSF, is predominantly expressed on professional APCs, including B cells, macrophages, DCs and a wide variety of human cancer cells. It is also expressed on a broad variety of non-immune cells, suggesting additional non-immunological functions. Both stimulatory and inhibitory properties have been identified but the ligand of B7-H3 has yet to be identified.
B7-H4
B7-H4 (B7 homolog 4), from the B7 family of the IgSF, is expressed on the surface of activated lymphocytes, macrophages, monocytes, DCs, epithelial cells, and bone marrow-derived mesenchymal stem cells, as well as numerous tumor cells. It is a negative regulator of T cell immunity that inhibits T cell proliferation, cytokine production, and cell cycle progression. Its ligand is still unknown.
LAG-3 and MHC-II
LAG-3 (Lymphocyte-activation gene 3) is expressed on activated T cells, NKs, B cells, and plasmacytoid DCs. Its main ligands are MHC-II molecules, which are constitutively expressed on professional immune APCs. LAG-3 binds MHC-II in a similar manner to CD4, but with higher affinity. It negatively regulates proliferation, activation, and homeostasis of T cells in a similar fashion to CTLA-4 and PD-1.
Tumor Necrosis Factor Receptor Superfamily (TNFRSF)
The TNFRSF is comprised of the homologs, and currently includes 29 receptors, which bind ligands via an extracellular cysteine-rich domain, and 19 ligands. Most TNF receptors form trimeric complexes in the plasma membrane upon activation and require an adaptor protein for downstream signaling. TNF receptors are mainly involved in apoptosis and inflammation, but can also participate in other signaling pathways, such as proliferation, survival and differentiation. Several of the members of this family contain cytoplasmic ‘death domains’ that are crucial for the initiation of an apoptotic response.
HVEM and LIGHT/BTLA/CD160
HVEM (TNFRSF14, Herpes Virus Entry Mediator) is a member of the TNF receptor superfamily that is widely expressed on APCs, endothelium and lymphocytes, especially on naïve T cells. It serves as a receptor to several ligands, including LIGHT, TNF-β, BTLA (B and T lymphocyte attenuator), and CD160. Expressed by activated T cells, NK cells, monocytes, granulocytes and immature dendritic cells, LIGHT functions as a costimulatory factor for the activation of lymphoid cells when binding to HVEM. In contrast to LIGHT, BTLA and CD160 inhibit of T cell activation when bound to HVEM. BTLA is expressed by splenic B and T cells, macrophages, NK cells and dendritic cells; whereas, CD160 is expressed by NK cells, NKT cells and certain subsets of T cells, as well as on the surface of all intestinal intraepithelial lymphocytes. [4]
OX40 and OX40L
OX40 (TNFRSF4) is expressed on activated T cells, NK cells, NKT cells, and neutrophils. It functions as a secondary co-stimulatory immune checkpoint. Its ligand, OX40L is generally expressed during inflammation is found on APCs, including dendritic cells, B cells and macrophages. OX40L is expressed on activated APCs, but is not found on resting APCs. OX40 signaling helps effectuate response to T cell activation by supporting the survival and expansion of activated T cells, and the establishment of T cell memory.
GITR and GITRL
GITR (Glucocorticoid Induced TNFR Related Protein, TNFRSF18) is constitutively expressed on Tregs (T-regulatory cells). On resting CD4+ and CD8+ cells, GITL is upregulated about 24 hours after antigen activation and remains more highly expressed for several days thereafter. GITR is also expressed on various peripheral blood leukocytes and stimulated monocytes. Its ligand, GITRL is highly expressed on activated APCs and endothelial cells at sites of inflammation. GITR appears to play a role in promoting T effector cell activity by inducing proliferation and supporting survival in T cells, while also suppressing Treg activity. [4]
CD27 and CD70
CD27 is a co-stimulatory molecule that is expressed on numerous T lymphocytes, and certain memory B cells and NK cells. Its ligand, CD70, can be expressed on activated lymphocytes and is highly elevated in some tumors. Ligation of CD27 is involved in the activation and survival of NK cells, the maintenance of T cell effector functions, the development of T cell memory, support of germinal center formation and B cell maturation, and the production of high affinity IgG antibodies. [4]
CD30 and CD153
CD30 (TNFRSF8) is expressed by activated, but not resting, T and B cells while its ligand, CD153, is expressed on activated T cells. CD30/CD153 ligation mediates pleiotropic effects including cell proliferation, activation, differentiation and apoptosis. CD30 is overexpressed in various hematological malignancies, and is found in the leukocytes of patients with chronic inflammatory and autoimmune diseases including lupus erythematosus, asthma and rheumatoid arthritis.
CD40 and CD154
CD40 (TNFRSF5) is expressed on the surface of APCs, including B cells, dendritic cells, and macrophages as well as on the surface of endothelial cells, epithelial cells and a variety of tumor cells. Its ligand, CD154, is expressed on activated T cells, platelets, and several other cell types. The activation of CD40 is involved in germinal center formation, B cell development, and immunoglobulin isotype switching. Interaction of CD40 with CD154 is critical to co-stimulation and immune regulation. The importance of CD40 has been implicated in the pathology of multiple cardiovascular diseases, such as atherosclerosis and atherothrombosis.
4-1BB and 4-1BBL
4-1BB (TNFRSF9) is mainly expressed on activated CD4+ and CD8+ T cells, activated B cells, dendritic cells, and natural killer cells; whereas, its ligand, 4-1BBL (TNFSF9), is mainly expressed on APCs, and activated B and T cells. Ligation of 4‑1BB on T cells and natural killer cells induces cell activation, promotes survival, and enhances effector functions [4].
References:
1. Ceeraz, S., Nowak, E. C., Burns, C. M., & Noelle, R. J. (2014). Immune checkpoint receptors in regulating immune reactivity in rheumatic disease. Arthritis research & therapy, 16(5), 469.
2. Chen, L., & Flies, D. B. (2013). Molecular mechanisms of T cell co-stimulation and co-inhibition. Nature Reviews Immunology, 13(4), 227-242.
3. Croft, M. (2003). Co-stimulatory members of the TNFR family: keys to effective T-cell immunity. Nature Reviews Immunology, 3(8), 609-620.
4. Mahoney, K. M., Rennert, P. D., & Freeman, G. J. (2015). Combination cancer immunotherapy and new immunomodulatory targets. Nature reviews Drug discovery,14(8), 561-584.
5. Márquez-Rodas, I., Cerezuela, P., Soria, A., Berrocal, A., Riso, A., González-Cao, M., & Martín-Algarra, S. (2015). Immune checkpoint inhibitors: therapeutic advances in melanoma. Annals of translational medicine, 3(18), 267-282.
6. Nguyen, L. T., & Ohashi, P. S. (2015). Clinical blockade of PD1 and LAG3 - potential mechanisms of action. Nature Reviews Immunology, 15(1), 45-56.
7. Pardoll, D. M. (2012). The blockade of immune checkpoints in cancer immunotherapy. Nature Reviews Cancer, 12(4), 252-264.
8. Sharpe, A. H. (2009). Mechanisms of costimulation. Immunological reviews, 229(1), 5-11.
9. Topalian, S. L., Drake, C. G., & Pardoll, D. M. (2015). Immune Checkpoint Blockade: A Common Denominator Approach to Cancer Therapy. Cancer cell, 27(4), 450-461.
Ligand |
Receptor |
|||
| Description | Catalog # | Description | Catalog # | |
| CD80 (B7-1) | CD28 | |||
| Recombinant Human B7-1 Fc | 310-32 | Recombinant Human sCD28 Fc | 310-34 | |
| CD86 (B7-2) | 4-1BB (CD137) | |||
| Recombinant Human B7-2 Fc | 310-33 | Recombinant Human 4-1BB Receptor | 310-15 | |
| OX40L (CD252) | Anti-Human 4-1BB Receptor | 500-P167G | ||
| Recombinant Human sOX40 Ligand | 310-28 | Biotinylated Anti-Human 4-1BB Receptor | 500-P167GBT | |
| 4-1BBL | Human 4-1BB Receptor Standard ABTS EDK | 900-K208 | ||
| Recombinant Human 4-1BB Ligand | 310-11 | ICOS (CD278) | ||
| Anti-Human 4-1BB Ligand | 500-P169 | Recombinant Human ICOS Fc | 310-39 | |
| Biotinylated Anti-Human 4-1BB Ligand | 500-P169BT | HVEM (CD270) | ||
| CD70 | Recombinant Human HVEM-Fc | 310-27 | ||
| Recombinant Human sCD27 Ligand | 310-30 | |||
ICOSL (B7-H2; CD275) |
||||
| Recombinant Human B7-H2 Fc | 310-37 | |||
| GITRL (AITRL) | ||||
| Recombinant Human AITRL | 310-22 | |||
| Anti-Human AITRL | 500-P244 | |||
| Biotinylated Anti-Human AITRL | 500-P244BT | |||
| CD153 | ||||
| Recombinant Human sCD30 Ligand | 450-42 | |||
| CD154 | ||||
| Recombinant Murine sCD40 Ligand | 315-15 | |||
| Recombinant Human sCD40 | 310-02 | |||
| Animal-Free Recombinant Human sCD40 | AF-310-02 | |||
| Anti-Human CD40 Ligand | 500-P142G | |||
| Biotinylated Anti-Human CD40 Ligand | 500-P142GBT | |||
| Human sCD40 Ligand Standard ABTS EDK | 900-K145 | |||
| Human sCD40 Ligand Mini ABTS EDK | 900-M145 | |||
| HVEML (LIGHT; CD258) | ||||
| Recombinant Human LIGHT | 310-09B | |||
| Anti-Human LIGHT | 500-P179 | |||
| Biotinylated Anti-Human LIGHT | 500-P179BT | |||
| Recombinant Murine LIGHT | 315-12 | |||
| Anti-Murine LIGHT | 500-P308 | |||
| Biotinylated Anti-Murine LIGHT | 500-P308BT | |||
Ligand |
Receptor |
|||
| Description | Catalog # | Description | Catalog # | |
| PD-L1 (B7-H1; CD274) | PD1 (CD279) | |||
| Recombinant Human PD-L1 Fc | 310-35 | Recombinant Human PD-1 Fc | 310-40 | |
| Anti-Human PD-L1 Fc | 500-P321 | CTLA4 (CD152) | ||
| Biotinylated Anti-Human PD-L1 Fc | 500-P321BT | Recombinant Human CTLA-4 Fc | 310-05 | |
| PD-L2 (B7-DC; CD273) | TIM3 (CD366) | |||
| Recombinant Human PD-L2 Fc | 310-38 | Recombinant Human TIM-3 Fc (coming soon) | 150-20 | |
| CD80 (B7-1) | HVEM (CD270) | |||
| Recombinant Human B7-1 Fc | 310-32 | Recombinant Human HVEM-Fc | 310-27 | |
| Anti-Murine B7-1 | 500-P128 | TMIGD2/CD28H | ||
| Biotinylated Anti-Murine B7-1 | 500-P128BT | Recombinant Human TMIGD2/CD28H Fc | 310-42 | |
| CD86 (B7-2) | ||||
| Recombinant Human B7-2 Fc | 310-33 | |||
| BTLA (CD272) | ||||
| Recombinant Human BTLA FC | 310-43 | |||
| B7-H7 | ||||
| Recombinant Human B7-H7 (coming soon) | 310-44 | |||
