Immunology Research Booklet
Immunology Research Related Cytokine Products
Introduction
The immune system is composed of a complex network of cells, tissues, and organs that function together as the body's defense against infectious organisms, diseases, and other invasive agents. Representing a duality of responsibilities, the immune system initiates the body's quick and efficient response to alien agents, while also distinguishing these threats from the body's healthy cells and working to avoid attacks against the host; a process known as autoimmunity.
The immune system is comprised of several different cell types that collectively serve to protect the body from bacterial, fungal, and viral infections, as well as from the growth and dispersal of tumor cells. The various cell types have distinct and specialized functions, such as engulfing bacteria, producing antibodies, and killing parasites, tumor cells and virally-infected cells.
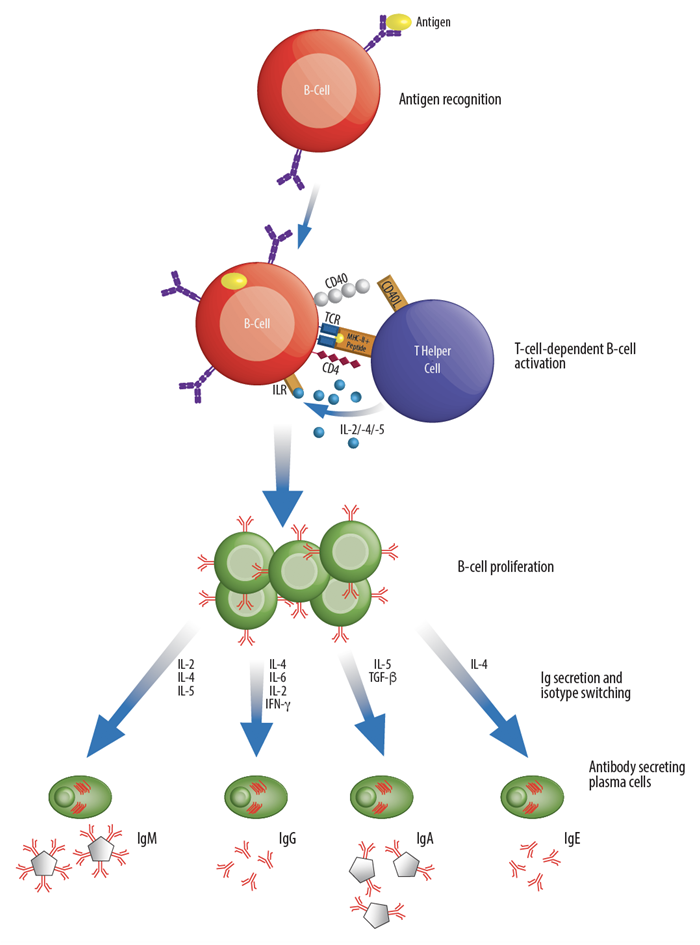
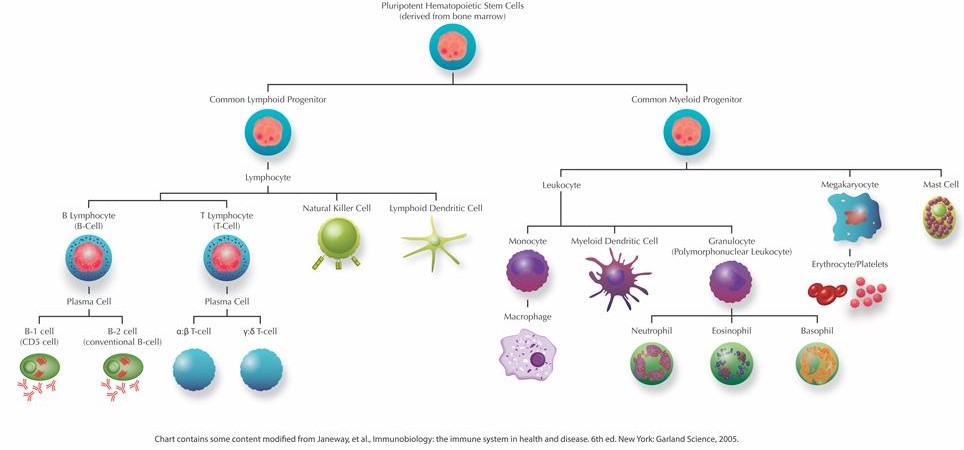
Generally, the immune system can be divided into two major layers of defense: innate immune responses and adaptive immune responses. The innate immune response represents an organism's immediate reaction to and generic barrier against infections. On the other hand, the adaptive immune response is specific to the invasive agent and can initiate antibody production, cell mediated responses, and immunological memory. While both innate and adaptive immunity depend upon the servitude of leukocytes, the adaptive response relies specifically upon three specialized leukocytes called lymphocytes: those being B-lymphocytes (B-cells), T-cells and Natural Killer Cells (NK cells). These three types of lymphocytes collectively define the adaptive immune response, however, they each have focused roles and function through distinct types of receptors. Whereas B-cells are responsible for the production of antibodies and in their mature form are referred to as plasma cells, T-cells can develop into effector cells in response to an activating antigen and are responsible for cell-mediated immunity. The functions of effector cells fall into one of three broad classes: killing, activation and regulation. For example, Cytotoxic T-cells serve the purpose of killing cells that have been infected with intercellular pathogens, such as viruses. While Helper T-cells provide essential intercellular signals that influence the behavior and activity of other immune cells (including B-cells and macrophages), Regulatory T-cells mediate the activity of other lymphocytes and help regulate immune responses. During the course of the immune response, a number of those B- and T-cells that have survived past infections can also serve to differentiate into the long-living lymphocytes, known as Memory cells, responsible for immunological memory.
Lymphocytes and other cells from the immune system, such as macrophages and dendritic cells, produce a large array of cell signaling proteins that are collectively referred to as cytokines, which are responsible for the intercellular communications necessary for the accurate and efficient performance of both innate and adaptive immune responses. Cytokines are proteins produced, usually as the result of an activating stimulus, by various cells of the body that induce signaling by binding to specific cell surface receptors. In general terms, cytokines are responsible for much of the activation and regulation of the body's response to disease and infection, and can directly affect the activity of most immune cells. Playing a crucial role in the functioning of lymphocytes, cytokines can serve to recruit other cells in the body's response to invasion and act to mediate normal cellular processes. Thus, deciphering the action of cytokines is central to understanding various aspects of the immune system.
The body produces several different classes of cytokines, including:
• Colony stimulating factors: cause proliferation and differentiation of specific target cells
• Growth and differentiation factors: subfamily of TGF-β like proteins that play an important role during prenatal and postnatal development, and the maintenance of various tissues
• Proinflammatory cytokines: promote systemic and site specific inflammation
• Chemokines: a group of structurally related cytokines that can induce chemotaxis of specific nearby cells
Our understanding of the immune system has advanced significantly in recent years, and it has become evident that cytokines play a central role in the activation and regulation of the immune response. This booklet describes a diverse number of commercially available cytokine products. By offering this wide array of cytokine reagents, it is PeproTech's goal to contribute to the continuing advances of immune system related research and the overall improvement of worldwide health.
Cells of the Immune System
B-Cells
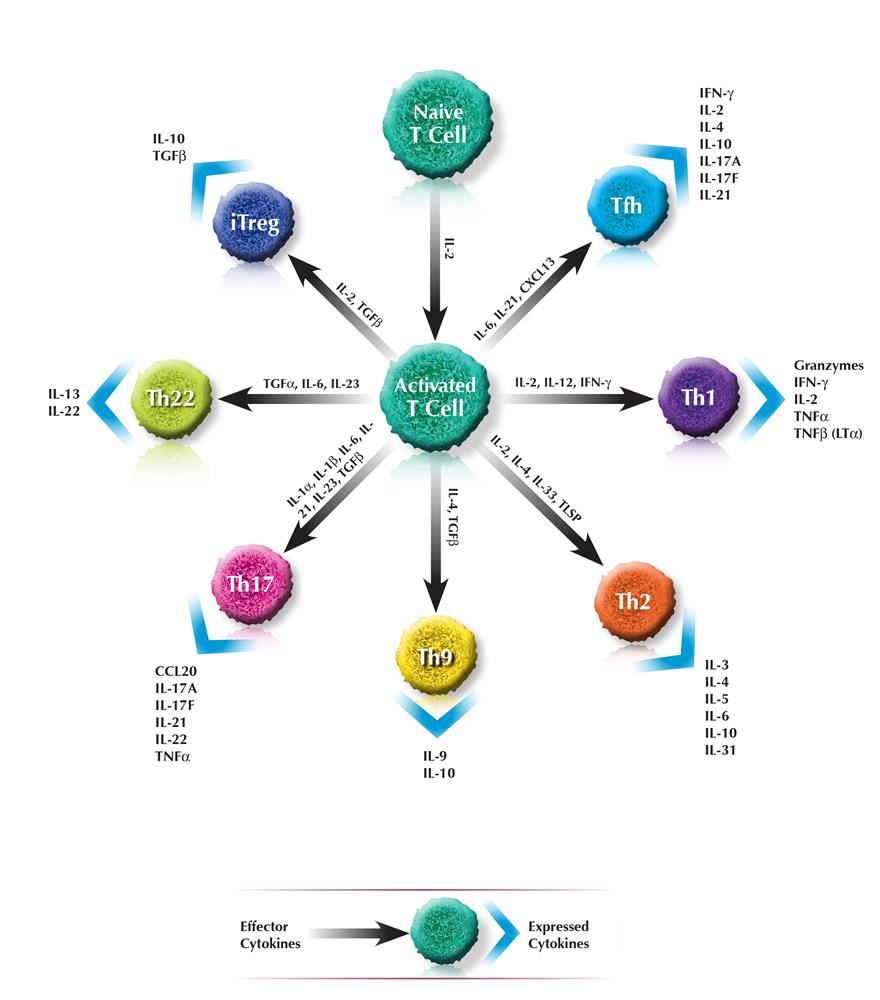
T-HELPER CELLS
Key Effector and expressed Cytokines related to T-helper cells
|
|
Th1 |
T-helper type 1 |
|
| Cytokines Associated with Th1 Cells | |||
|
• IFN-γ • IL-2 • IL-10 |
• IL-12 • IL-18 • IL-27 • TNF-α • TNF-β |
||
| Disease/Disorder Association | |||
|
• Inflammatory bowel disease • Multiple schlerosis • Rheumatoid arthritis • Type I diabetes |
|||
| Th2 |
T-helper type 2 |
 |
|
|
|
Cytokines Associated with Th2 Cells | ||
|
• IFN-γ • IL-2 • IL-4 • sIL-4Rα • IL-5 • IL-6 • IL-9 |
• IL-13 • IL-21 • IL-25 • IL-31 • IL-33 • TSLP |
||
| Disease/Disorder Association | |||
|
• Asthma • Chronic allergic inflammation |
|||
 |
Th9 |
T-helper type 9 |
|
| Cytokines Associated with Th9 Cells | |||
|
• IL-4 • sIL-4α • IL-9 |
• IL-10 |
||
| Disease/Disorder Association | |||
|
• Airway remodeling autoimmune disease • Chronic allergic inflammation |
|||
| Th17 |
T-helper type 17 |
 |
|
|
|
Cytokines Associated with Th17 Cells | ||
|
• IL-1β • IL-6 • sIL-6Rα • IL-17E • IL-17F |
• IL-21 • IL-22 • IL-23 • IL-26 • MIP-3α • TNF-α |
||
| Disease/Disorder Association | |||
|
• Inflammatory bowel disease • Multiple schlerosis • Rheumatoid arthritis |
|||
|
|
Tfh |
T Follicular helper |
|
| Cytokines Associated with Tfh Cells | |||
|
• BCA-1 • IFN-γ • IL-2 • IL-4 • IL-6 |
• sIL-6Rα • IL-10 • IL-12 • IL-21 • IL-17A • IL-17F |
||
| Disease/Disorder Association | |||
|
• Autoimmune diseases • Cancers |
|||
| Treg |
Regulatory T |
 |
|
|
|
Cytokines Associated with Treg Cells | ||
|
• AITRL • IL-2 • IL-10 |
• IL-12 • IL-35 |
||
| Disease/Disorder Association | |||
|
• Autoimmunity • Inflammatory disorders |
|||
 |
Th22 |
T-helper type 22 |
|
| Cytokines Associated with Th22 Cells | |||
|
• IL-6 • sIL-6Rα • IL-10 • IL-13 • IL-21 |
• IL-22 • IL-26 • TNF-α |
||
| Disease/Disorder Association | |||
|
• Allergic contact dermatitis • Atopic eczema • Psoriasis |
|||
| NKT |
Natural Killer T |
 |
|
|
|
Cytokines Associated with NKT Cells | ||
|
• GM-CSF • IFN-γ • IL-10 |
• IL-13 • TNF-α |
||
| Disease/Disorder Association | |||
|
• Asthma • Atherosclerosis • Cancers |
|||
 |
CTLs |
Cytotoxic T |
|
| Cytokines Associated with Cytotoxic T Cells | |||
|
• IFN-γ |
• TNF-α • TNF-β |
||
| Disease/Disorder Association: | |||
|
• Arthritis • Liver injury due to HBV |
|||
| B-Cells |  |
||
|
|
Cytokines Associated with B-Cells | ||
|
• BAFF • BCA-1 • BCMA • sCD23 • Exodus-2 • HVEM • ICAM-1 • IFN-α • IFN-γ |
• IL-2 • sIL-2Rα • IL-4 • sIL-4Rα • IL-5 • IL-6 • sIL-6Rα • IL-10 • IL-17A • SDF-1α • SDF-1β • TACI • TSLP |
||
| Disease/Disorder Association | |||
|
• Autoimmune diseases • Cancer • Diabetes • Grave's Disease • Immunodeficiencies • Inflammatory Bowel disease |
|||
 |
Dendritic Cells (DCs) |
|||
| Cytokines Associated with Dendritic Cells | ||||
|
• AITRL • Eotaxin • Exodus-2 • GM-CSF • I-309 • ICAM-1 • IFN-α • IFN-β |
• IFN-γ • IL-2 • IL-3 • IL-4 • IL-6 • IL-8 • IL-12 • IL-13 • IL-15 • IL-21 • IL-27 • IP-10 • I-TAC |
• MCPs • MDC • MIG • MIP-1α • MIP-1β • MIP-3α • MIP-3β • RANTES • SDF-1 • TLR3 • TNF-α • TSLP |
||
| Disease/Disorder Association | ||||
|
• Allergy • HIV infection • Inflammatory bowel diseases |
||||
| Natural Killer Cells (NKs) |
 |
||
|
|
Cytokines Associated with NK Cells | ||
|
• IFN-γ |
• IL-2 • IL-7 • IL-10 • IL-12 • IL-15 • sTRAIL |
||
| Disease/Disorder Association | |||
|
• Cancer • Possibly HIV |
|||

|
Macrophages | |||||
| Cytokines Associated with Macrophages | ||||||
|
• CD40 • G-CSF • GM-CSF • IFNs • IL-1β • IL-1RA • IL-3 • IL-4 • IL-6 • IL-8 |
• IL-10 • IL-12 • IL-18 • IL-27 • IP-10 • I-TAC • LIF • MCP-1 • MCP-2 • MCP-3 • M-CSF |
• MIF • MIG • MIP-1α • MIP-1β • MIP-2 • RANTES • TNF-α |
||||
| Disease/Disorder Association | ||||||
|
• Cancer • Chikungunya • Heart Disease • HIV Infection • Lei Shmaniasis • Obesity • Tuberculosis |
||||||
| Monocytes |  |
||
|
|
Cytokines Associated with Monocytes | ||
|
• BRAK • C10 • HCC-1 • IL-4 • IL-19 • IL-20 • IL-24 • LAG-1 • LD78β • LEC |
• LIGHT • MCPs • MIP-1α • MIP-1β • MIP-3α • MIP-3β • MIP-5 • RANTES • SDF-1α • SDF-1β • TNF-α |
||
| Disease/Disorder Association | |||
|
• Cancer • Chronic Inflammation • Hyperadrenocorticism • Immune-related disease • Pyogranulomatous disease • Red Cell Regeneration • Sarcoidosis • Viral Fever |
|||
 |
Neutrophils | ||||
| Cytokines Associated with Neutrophils | |||||
|
• ENA-78 • GCP-2 • GROα • GRO-β • GRO-γ • IL-8 • Lungkine |
• MIP-3α • MIP-3β • MIP-5 • NAP-2 • SDF-1α • SDF-1β |
||||
| Disease/Disorder Association | |||||
|
• Aplastic Anemia • Inflammation • Leukemia • Pulmonary Emphysema |
|||||
| Eosinophils |  |
||
|
|
Cytokines Associated with Eosinophils | ||
|
• Eotaxin • HCC-1 • MCP-3 |
• MCP-4 • MIP-1α • MIP-5 • RANTES |
||
| Disease/Disorder Association | |||
|
• Addison's disease • Eosinophilic Esophagitis • Hodgkin's disease • Reflux Esophagitis • Rheumatoid Arthritis • Skin diseases |
|||
 |
Basophils | ||||
| Cytokines Associated with Basophils | |||||
|
• Eotaxin |
• MCPs • RANTES |
||||
| Disease/Disorder Association | |||||
|
• Cancer • Inflammation/Allergy |
|||||
Cytokines Relevent to Immune Research
4-1BBL, a member of the TNF superfamily, is expressed in B cells, dendritic cells, activated T cells and macrophages. 4-1BBL binds to its receptor 4-1BB, and provides a co-stimulatory signal for T cell activation and expansion. The human 4-1BBL gene codes for a 254 amino acid type II transmembrane containing a 28 amino acid cytoplasmic domain, a 21 amino acid transmembrane domain, and a 205 amino acid extracellular domain. The soluble form of 4-1BBL contains the TNF-like portion of the extracellular domain of 4-1BBL.
4-1BB receptor, a member of the TNFR superfamily of receptors, is mainly expressed on the surface of T cells, but also found in B cells, monocytes, and various transformed cell lines. 4-1BB receptor binds to 4-1BBL to provide a co-stimulatory signal for T lymphocytes. Signaling by 4-1BB receptor has been implicated in the antigen-presentation process and generation of cytotoxic T cells. The human 4-1BB receptor gene codes for a 255 amino acid type I transmembrane protein containing a 17 amino acid N-terminal signal sequence, a 169 amino acid extracellular domain, a 27 amino acid transmembrane domain and a 42 amino acid cytoplasmic domain.
Activin A is a TGF-β family member that exhibits a wide range of biological activities, including regulation of cellular proliferation and differentiation, and promotion of neuronal survival. Elevated levels of Activin A in human colorectal tumors and in postmenopausal women have been implicated in colorectal and breast cancers, respectively. The biological activities of Activin A can be neutralized by inhibins and by the diffusible TGF-β antagonist, follistatin. Activin A binds to the two forms of activin receptor type I (Act RI-A and Act RI-B) and two forms of activin receptor type II (Act RII-A and Act RII-B). Activins are homodimers or heterodimers of different b subunits. They are produced as precursor proteins with an amino terminal propeptide that is cleaved to release the C-terminal bioactive ligand.
Activin B is a TGF-β family member that exhibits a wide range of biological activities, including regulation of embryogenesis, osteogenesis, hematopoiesis, reproductive physiology and hormone secretion from the hypothalamic, pituitary and gonadal glands. Activin B, like certain other members of the TGF-β family, signals through the ActRII receptor (Activin Receptor type II). Activins are homodimers or heterodimers of different β subunits. They are produced as precursor proteins with an amino terminal propeptide that is cleaved to release the C-terminal bioactive ligand.
Adiponectin is an adipose-derived secreted protein containing 236 amino acid residues. It is relatively abundant in humans and rodents, accounting for about 0.01% of total plasma protein. The circulating levels of adiponectin are decreased under conditions of obesity, insulin resistance, and type II diabetes. Disruption of adiponectin in mice causes insulin resistance and neointimal formation. Conversely, administration of recombinant adiponectin suppresses hepatic glucose production, and reverses insulin resistance associated with both lipoatrophy and obesity. The protective role of adiponectin is attributed to its anti-inflammatory properties (e.g. ability to suppress expression of TNF-α and class A scavenger receptor in macrophages).
AITRL, a member of the TNF superfamily, is expressed in endothelial cells, and signals through the AITR receptor. AITRL regulates T-cell proliferation and survival, and effectuates the interaction between T lymphocytes and endothelial cells. The AITRL gene codes for a type II transmembrane protein comprised of 177 amino acids, including a 28 amino acid cytoplasmic region, a 21 amino acid transmembrane domain and a 128 amino acid extracellular domain.
Amphiregulin is an EGF-related growth factor that signals through the EGF/TGF-α receptor, and stimulates growth of keratinocytes, epithelial cells and some fibroblasts. Amphiregulin also inhibits the growth of certain carcinoma cell lines. Synthesized as a transmembrane protein, Amphiregulinís extracellular domain is proteolytically processed to release the mature protein. There are 6 conserved cysteine residues, which form 3 intramolecular disulfide bonds essential for biological activity.
Angiopoietin-1 (ANG-1) is a secreted ligand for Tie-2, a tyrosine-kinase receptor expressed primarily on vascular endothelial cells and early hematopoietic cells. ANG-1/Tie-2 signaling promotes angiogenesis during the development, remodeling, and repair of the vascular system. Transgenic mice lacking expression of either ANG-1 or Tie-2 fail to develop a fully functional cardiovascular system and die before birth. Postnatally, the angiogenic activity of ANG-1/Tie-2 is required during normal tissue repair and remodeling of the female endometrium in the menstrual cycle. ANG-1/Tie-2 signaling appears to be regulated by Angiopoietin-2 (ANG-2), a natural antagonist for Tie-2 that exerts its effects through an internal autocrine loop mechanism. In addition to suppressing endothelial cell activation by inhibiting the expression of adhesion and inflammatory molecules, Ang-1 enhances endothelial cell survival and capillary morphogenesis, and lessens capillary permeability. As such, ANG-1 has potential to become an effective therapeutic agent for treating various endothelium disorders, including several severe human pulmonary diseases. The efficacy of cell-based Ang-1 gene therapy for acute lung injury (ALI) has recently been studied in a rat model of ALI. The results of this study show that such therapy can markedly improve lung condition and suggest that Ang-1 therapy may represent a potential new strategy for the treatment and/or prevention of acute respiratory distress injury (ARDI), a significant cause of morbidity and mortality in critically ill patients.
ANG-2 binds to the endothelial cell specific receptor Tie-2, but, in contrast to ANG-1, does not induce tyrosine phosphorylation. Consequently, ANG-2 modulates ANG-1 activation of Tie-2 and, depending on the physiological and biochemical environment, can act either as an agonist or antagonist of Tie-2 induced angiogenesis. The signaling interactions of ANG-1, ANG-2 and Tie-2, along with less characterized ANG-3 and ANG-4, are required for embryonic and adult angiogenesis. Physiologically, ANG-1 and ANG-2 are associated with sprouting, tube formation, and structural integrity of newly formed blood vessels. Mature human ANG-2 is a secreted protein containing 480 amino acid residues. ANG-2 is composed of an alpha-helix-rich "coiled coil" N-terminal domain and fibrinogen-like C-terminal domain. ANG-2 exists predominantly in the form of a disulfide-linked dimer.
ApoA-I is a 29.0 kDa protein produced in the liver and intestine, and secreted as the predominant constituent of nascent high density lipoprotein (HDL) particle. ApoA-I, which is found exclusively in HDL, has a unique ability to capture and solubilize free cholesterol. This ApoA-I ability enables HDL to remove excess peripheral cholesterol, and return it to the liver for recycling and excretion. This process, called reverse cholesterol transport, is thought to inhibit atherogenesis. For this reason, HDL is also known as the "good cholesterol." The therapeutic potential of ApoA-I has been recently assessed in patients with acute coronary syndromes, using a recombinant form of a naturally occurring variant of ApoA-I (called ApoA-I Milano). The availability of recombinant normal ApoA-I should facilitate further investigation into the potential usefulness of ApoA-I in preventing atherosclerotic vascular diseases.
ApoE belongs to a group of proteins that bind reversibly with lipoprotein and play an important role in lipid metabolism. In addition to facilitating solubilization of lipids, these proteins help to maintain the structural integrity of lipoproteins, serve as ligands for lipoprotein receptors, and regulate the activity of enzymes involved in lipid metabolism. Significant quantities of ApoE are produced in the liver and brain, and to some extent in almost every organ. ApoE is an important constituent of all plasma lipoproteins. Its interaction with specific ApoE receptor enables uptake of chylomicron remnants by liver cells, which is an essential step during normal lipid metabolism. It also binds with the LDL receptor (apo B/E). Defects in ApoE are a cause of hyperlipoproteinemia type III. ApoE exists in three major isoforms; E2, E3, and E4, which differ from one another by a single amino-acid substitution. Compared with E3 and E4, E2 exhibits the lowest receptor binding affinity. E2 allele carriers had significantly lower levels of total cholesterol, low-density lipoprotein cholesterol, and non-high-density lipoprotein cholesterol, as well as increased ApoE levels. E3 is the most common isoform and is present in 40-90% of the population. Individuals heterozygous for the ApoE4 allele are at higher risk of late-onset Alzheimerís disease.
Serum amyloid A proteins (SAA) represents a family of apolipoproteins that circulates in association with high-density lipoproteins (HDL). The level of Apo-SAA, normally 1-5 µg/ml in plasma, increases 500-1000 fold within 24 hours of an inflammatory stimulus and, under these conditions, is the most abundant HDL apolipoprotein. The human SAA gene codes for a 122 amino acid nonglycosylated polypeptide, which contains an 18 amino acid N-terminal sequence. Recombinant Apo-SAA is a consensus SAA molecule corresponding to human Apo-SAA1α, except for the presence of an N-terminal methionine, the substitution of asparagine for aspartic acid at position 60, and arginine for histidine at position 71 (the latter two substituted residues are present in Apo-SAA2β).
APRIL, a member of the TNF superfamily, is expressed in monocytes, macrophages, certain transformed cell lines, certain cancers of the colon, and lymphoid tissues. APRIL, along with another TNF family member, BAFF, competes for two receptors, TACI and BCMA. APRIL has the ability to stimulate proliferation of various tumor cell lines, including Jurkat T cells and MCF-7 carcinoma cells. Like BAFF, APRIL also stimulates the proliferation of B and T cells. The human APRIL gene codes for at least four alternatively spliced transcriptional variants, which give rise to different isoforms of the APRIL precursor protein. All isoforms can be cleaved by the protease, furin, to release a soluble C-terminal fragment, which comprises the TNF-like receptor binding of the APRIL precursor.
Proteases (also called Proteolytic Enzymes, Peptidases, or Proteinases) are enzymes that hydrolyze the amide bonds within proteins or peptides. Most proteases act in a specific manner, hydrolyzing bonds at, or adjacent to specific residues, or a specific sequence of residues contained within the substrate protein or peptide. Proteases play an important role in most diseases and biological processes, including prenatal and postnatal development, reproduction, signal transduction, the immune response, various autoimmune and degenerative diseases, and cancer. They are also an important research tool, frequently used in the analysis and production of proteins. Arg-C specifically cleaves at the carboxyl side of Arginine residues. Arg-C has a sulfhydryl requirement; it is activated by dithiothreitol, cysteine, or other sulfhydryl-containing reagents. The presence of calcium ions is essential. The enzyme is inhibited by oxidizing agents and sulfhydryl reactants, and by Co2+, Cu2+, Cd2+, and heavy metal ions.
Artemin is a disulfide-linked homodimeric neurotrophic factor structurally related to GDNF, Artemin, Neurturin and Persephin. These proteins belong to the cysteine knot superfamily of growth factors that assume stable dimeric protein structures. Artemin, GDNF, Persephin and Neurturin all signal through a multicomponent receptor system, composed of RET (receptor tyrosine kinase) and one of the four GFRα (α1-α4) receptors. Artemin prefers the receptor GFRα3-RET, but will use other receptors as an alternative. Artemin supports the survival of all peripheral ganglia, such as sympathetic, neural crest and placodally-derived sensory neurons, and dopaminergic midbrain neurons. The functional human Artemin ligand is a disulfide-linked homodimer of two 12.0 kDa polypeptide monomers. Each monomer contains seven conserved cysteine residues, one of which is used for interchain disulfide bridging and the others are involved in intramolecular ring formation known as the cysteine knot configuration.
B7-1 and B7-2 are transmembrane glycoproteins of the immunoglobulin superfamily that are expressed, along with the receptors CD28 and CTLA4, by antigen-presenting cells, and along with these receptors, constitute crucial costimulatory pathways for T and B cell regulatory responses. As members of the B7 family, B7-1 and B7-2 play principal roles in immunity, activating immune response and maintaining immune tolerance through engagement with CD28 and CTLA4. Co-stimulatory signals generated by B7-1 and B7-2 interactions with CD28 serve to stimulate T cell activation and prevent anergy through the amplification of T cell receptor (TCR) signaling. In contrast, interactions of the ligands with CTLA-4 serves to maintain T cell homeostasis and self-tolerance through the disruption of stimulatory signaling from B7 isoform bound CD28 complexes, and by inducing powerful inhibitory signals in T cells. B7-1 plays an important role in immune response through its amplification and regulation of T cell activity at peripheral inflammation sites. B7-1, like CTLA-4, is, however, only poorly expressed on resting dendritic cells, and its up-regulation is, therefore, considerably delayed upon immune activation. Conversely, B7-2 and CD28 are constitutively expressed by resting hematopoietic and T cells, respectively, and as a result are able to rapidly induce up-regulation upon immune activation, making them critical to the early costimulatory singling of immune response. Both B7-1 and B7-2 have been shown to demonstrate co-stimulatory activity in T-cell proliferationsin vitrosand elicit enhanced antitumor immune responses in vivo.
B7-H2, or inducible costimulator-ligand (ICOSL), is a transmembrane, co-stimulatory ligand of the T cell-specific surface receptor Inducible T-cell costimulator (ICOS) that belongs to the B7 family and immunoglobulin superfamily, along with B7-1, B7-2, PD-L1 (B7-H1) and PD-L2. Whereas expression of inducible B7-1 and B7-2 is largely confined to specialized antigen-presenting cells of lymphoid tissues, B7-H2 expression occurs constitutively in hematopoietic and non-hematopoietic cells of peripheral organs. This striking difference in expression indicates that these three B7 ligands may enable temporally and spatially-specific regulation of T cell response through non-competitive CD28 interaction; marking a unique function of B7-H2 in immune reactions of nonlymphoid organs in which T cells have migrated to peripheral tissues having only limited expression of B7-1 and B7-2. Expression of B7-H2 has been shown to be differentially regulated by both TNF-α and IL-1β, and inducible to a lesser extent by CD40 or lipopolysaccharide stimulation. B7-H2ís binding to ICOS on activated T cells results in both positive and negative effects on immune response, including its own downregulation. As a member of the immunoglobulin superfamily, B7-H2 is crucially involved in inflammatory immune reactions and the control of excessive immune response; however, unlike B7-1 and B7-2, B7-H2 has not been shown to influence immunity through interaction with CTLA-4, and has only been shown to have restricted interaction with CD28. Interaction of B7-H2 with ICOS has been identified as a critical event in the immunosuppression of tumor-associated memory CD4+ T cells, and has been linked to various auto-immune disorders.
BAFF, a member of the TNF superfamily of ligands, is expressed in T cells, macrophages, monocytes and dendritic cells. BAFF is involved in stimulation of B and T cell function, and is an important survival and maturation factor for peripheral B cells. BAFF signals through three different TNF receptors, TACI, BCMA and BAFF-R. The human BAFF gene codes for a 285 amino acid type II transmembrane protein containing a 46 amino acid cytoplasmic domain, a 21 amino acid transmembrane domain, and a 218 amino acid extracellular domain.
BAFF Receptor (BAFFR), a member of the TNFR superfamily, is highly expressed in the spleen, lymph nodes, and resting B cells, and to some extent in activated B cells, resting CD4+ cells and peripheral blood leukocytes. BAFFR is a type III transmembrane protein that binds with high specificity to BAFF (TNFSF13B). BAFFR/BAFF signaling plays a critical role in B cell survival and maturation.
BCA-1/BLC, a CXC chemokine, is expressed in the liver, spleen, lymph nodes, appendix and stomach. It exerts its activities through its only receptor, CXCR5. BCA-1/BLC is a potent chemoattractant for B lymphocytes, and induces weak chemotactic response in T cells and macrophages. It manifests no activity on neutrophils and monocytes.
BCMA, a member of the TNF receptor superfamily, binds to BAFF and APRIL. BCMA is expressed on mature B-cells and other B-cell lines, and plays an important role in B-cell development, function and regulation. BCMA also has the capability to activate NF-kB and JNK. The human BCMA gene codes for a 184 amino acid type I transmembrane protein, which contains a 54 amino acid extracellular domain, a 23 amino acid transmembrane domain, and a 107 amino acid cytoplasmic domain.
BD-1 / BD-2 / BD-3 / BD-4 / BD-5
Defensins (alpha and beta) are cationic peptides with a broad spectrum of antimicrobial activity that comprise an important arm of the innate immune system. The β-defensins are distinguished from the b-defensins by the pairing of their three disulfide bonds. To date, six human β-defensins have been identified; BD-1, BD-2, BD-3, BD-4, BD-5 and BD-6. β-defensins are expressed on some leukocytes and at epithelial surfaces. In addition to their direct antimicrobial activities, they can act as chemoattractants towards immature dendritic cells and memory T cells. The β-defensin proteins are expressed as the C-terminal portion of precursors, and are released by proteolytic cleavage of a signal sequence and, in some cases, a propeptide sequence. b-defensins contain a six-cysteine motif that forms three intra-molecular disulfide bonds.
Betacellulin is an EGF-related polypeptide growth factor that signals through the EGF receptor. It is produced in several tissues, including the pancreas, small intestine, and in certain tumor cells. Betacellulin is a potent mitogen for retinal pigment epithelial cells and vascular smooth muscle cells. Human Betacellulin is initially synthesized as a glycosylated 32.0 kDa transmembrane precursor protein, which is processed by proteolytic cleavage to produce the mature sequence.
BMPs (Bone Morphogenetic Proteins) belong to the TGF-b superfamily of structurally related signaling proteins. BMP-2 is a potent osteoinductive cytokine, capable of inducing bone and cartilage formation in association with osteoconductive carriers such as collagen and synthetic hydroxyapatite. In addition to its osteogenic activity, BMP-2 plays an important role in cardiac morphogenesis, and is expressed in a variety of tissues, including lung, spleen, brain, liver, prostate ovary and small intestine. The functional form of BMP-2 is a 26 kDa protein composed of two identical 114 amino acid polypeptide chains linked by a single disulfide bond. Each BMP-2 monomer is expressed as the C-terminal part of a precursor polypeptide, which also contains a 23 amino acid signal sequence for secretion, and a 259 amino acid propeptide. After dimerization of this precursor, the covalent bonds between the propeptide (which is also a disulfide-linked homodimer) and the mature BMP-2 ligand are cleaved by a furin-type protease.
TGF-β family members are key modulators of cell proliferation, differentiation, matrix synthesis, and apoptosis. As implied by their name, BMPs initiate, promote, and regulate the development, growth, and remodeling of bone and cartilage. In addition to this role, BMPs are also involved in prenatal development and postnatal growth, remodeling and maintenance of a variety of other tissues and organs. BMP-3 is abundantly found in adult bone and, to a lesser extent, fetal cartilage. BMP-3 inhibits osteogenesis and bone formation by activating a signaling cascade that antagonizes the signaling of pro-osteogenic BMPs.
Bone morphogenetic proteins (BMPs) constitute a subfamily within the TGF-β superfamily of structurally related signaling proteins. Members of this superfamily are widely distributed throughout the body, and are involved in diverse physiological processes during both pre- and postnatal life. Like BMP-7, BMP-4 is involved in the development and maintenance of bone and cartilage. Reduced expression of BMP-4 is associated with a number of bone diseases, including the heritable disorder Fibrodysplasia Ossificans Progressiva.
TGF-β family members are key modulators of cell proliferation, differentiation, matrix synthesis, and apoptosis. As implied by their name, BMPs initiate, promote, and regulate the development, growth, and remodeling of bone and cartilage. In addition to this role, BMPs are also involved in prenatal development and postnatal growth, remodeling, and maintenance of a variety of other tissues and organs. BMP-5 is expressed in the nervous system, lungs and liver. It is a known regulator for dendritic growth in sympathetic neurons. BMP-5 is a 454 amino acid precursor protein that is cleaved to release the biologically active C-terminal mature protein.
TGF-β family members are key modulators of cell proliferation, differentiation, matrix synthesis, and apoptosis. As implied by their name, BMPs initiate, promote, and regulate the development, growth, and remodeling of bone and cartilage. In addition to this role, BMPs are also involved in prenatal development and postnatal growth, remodeling, and maintenance of a variety of other tissues and organs. Increasing evidence indicates that BMP-Smad signaling has a tumor suppressing activity, and that BMPs can inhibit tumor growth. BMP-6 is abnormally expressed in breast cancer cell lines, however, its function in promoting breast cancer development is unknown. The mature and functional form of BMP-6 is a homodimer of two identical 139 amino acid polypeptide chains linked by a single disulfide bond. Each monomer is expressed as the C-terminal part of a precursor polypeptide, which contains a 20 amino acid signal peptide and a 354 amino acid propeptide. This precursor undergoes intracellular dimerization, and upon secretion it is processed by a furin-type protease.
TGF-β family members are key modulators of cell proliferation, differentiation, matrix synthesis, and apoptosis. As implied by their name, BMPs initiate, promote, and regulate the development, growth, and remodeling of bone and cartilage. In addition to this role, BMPs are also involved in prenatal development and postnatal growth, remodeling, and maintenance of a variety of other tissues and organs. BMP-7, also known as osteogenic protein-1 or OP-1, is a potent bone inducing agent, which in the presence of an appropriate osteoconductive carrier (e.g. collagen sponge or synthetic hydroxyapatite) can be used in the treatment of bone defects. A bone-graft substitute, called OP-1TM simplant, made of recombinant human BMP-7 associated with bovine bone-derived collagen, has recently been approved by the FDA as a device for treating critical-size bone fractures. The potential use of BMP-7 in dental reconstructive surgeries is currently under investigation.
BMP-13 is expressed in hypertrophic chondrocytes during embryonic development of long bones. Continued postnatal expression of BMP-13 in articular cartilage suggests that it plays a regulatory role in the growth and maintenance of articular cartilage. Adenovirus-mediated BMP-13 gene transfer to rabbit bone marrow stem cells have been reported to augment periosteal repair of osteochondral defects. The functional form of BMP-13/CDMP-2 is a disulfide-linked homodimer of two 120 amino-acid polypeptide chains. This 27.5 kDa protein is obtained by proteolytic processing of a biologically inactive precursor protein of 97.7 kDa.
Breast and Kidney-expressed chemokine (BRAK) is a CXC chemokine expressed in normal tissue in the absence of inflammatory stimuli, and infrequently expressed in cancer cell lines. BRAK is known to be a highly selective monocyte chemoattractant. However, main function and receptor selectivity is unknown at this time. BRAK contains the four highly conserved cysteine residues present in CXC chemokines. The sequence of the mature protein consists of 87 amino acid residues, and is approximately 30% homologous to the sequences of MIP-2 alpha and beta.
C1 inhibitor is a member of the serpin family of structurally related proteins, and is the primary regulator of the immune complement system. C1 inhibitor is a protease inhibitor that functions to inhibit the complement system in order to prevent over-activation or spontaneous activation. Inhibition is achieved by binding to and irreversibly inhibiting the C1r and C1s proteases of the C1 complex, which has the effect of shutting down all subsequent downstream events in the complement activation cascade. C1 inhibitor can also inhibit various other proteases, including Kallikrein, Factor XIa, and Factor XIIa. Deficiencies in C1 inhibitor are the primary cause of hereditary angioedema (HAE, hereditary angioneurotic edema), a disease characterized by edema in the respiratory and gastrointestinal tracts. In certain clinical situations, the direct administration of C1 inhibitor can be used to treat HAE and certain other conditions.
Murine C10 belongs to the CC chemokine family and is expressed in myelopoietic bone marrow cultures when stimulated with GM-CSF, M-CSF, IL-3 or IL-4. It signals primarily through the CCR1 receptor. C10 is chemotactic for B cells, CD4+ T cells, monocytes and NK cells and also exhibits powerful suppressive activity on colony formation by different lineages of hematopoietic progenitors. The C10 contains the four highly conserved cysteine residues present in CC chemokines. The mature protein contains 95 amino acid residues.
Complement 5a (C5a) is an enzymatically generated glycoprotein belonging to the anaphylatoxin family of structurally and functionally related proteins. Generated upon the activation of the complement system, C5a, together with C4a, C3a, and the membrane attack complex (C5b-9), functions as a central player in host defense by inducing smooth muscle cell contraction, increased vascular permeability, and histamine release from mast cells and basophilic leukocytes through cell degranulation. In addition to acting as a direct mediator of localized inflammatory response, C5a also initiates both the synthesis and release of IL-8 from monocytes and bronchial epithelial cells, stimulates the proliferation of neurons and hepatocytes, and functions as a potent chemoattractant. Where C5a deficiency, a rare defect of the complement pathway caused by the mutation of the C5a gene, is associated with susceptibility to severe infections, excessive C5a activation has been linked to liver fibrosis, sepsis, adult respiratory distress syndrome, rheumatoid arthritis, Alzheimerís disease, and ischemic heart disease. Human C5a shares 60% and 54% sequence identity to mouse and rat C5a, respectively. The human C5 gene encodes a 1,676 amino acid glycoprotein that is comprised of a disulfide-linked C5 alpha and a C5 beta chain, the former of which contains the active, 74 amino acid C5a anaphylatoxin chain.
Proteases (also called Proteolytic Enzymes, Peptidases, or Proteinases) are enzymes that hydrolyze the amide bonds within proteins or peptides. Most proteases act in a specific manner, hydrolyzing bonds at, or adjacent to, specific residues or a specific sequence of residues contained within the substrate protein or peptide. Proteases play an important role in most diseases and biological processes including prenatal and postnatal development, reproduction, signal transduction, the immune response, various autoimmune and degenerative diseases, and cancer. They are also an important research tool, frequently used in the analysis and production of proteins. Carboxypeptidase-B sequentially cleaves C-terminal K and R residues.
CT-1 is a member of the IL-6 family of cytokines which also includes LIF, CNTF, OSM (Oncostatin M), IL-11, IL-6 and possibly NNT-1/BSF-3. CT-1 is a pleiotropic cytokine which is expressed in various tissues including the adult heart, skeletal muscle, ovary, colon, prostate and fetal lung and signals through the LIF receptor and the gp130 receptor subunit. CT-1 has the ability to induce cardiac myocyte hypertrophy, and enhances the survival of cardiomyocyte and different neuronal populations. Biologically active human CT-1 is synthesized as a 201 amino acid polypeptide lacking a hydrophobic N-terminal secretion signal sequence.
Cluster determinant 4 (CD4), a type I transmembrane glycoprotein of the immunoglobulin family of receptors, plays an integral role in signal transduction and T-cell differentiation, development and activation. CD4 is constitutively expressed on the surface of various immune cells, including monocytes, macrophages, eosinophils, dendritic cells, and most prominently T-lymphocytes, where it functions as an essential co-receptor and co-ligand for T-cell receptor (TCR) and major histocompatibility complex class II (MHC-II) molecules. Ligation by MHC-II molecules on the surface of antigen-presenting cells can serve to influence adaptive immunity by facilitating helper T-cell activation and macrophage differentiation, while ligation by pro-inflammatory cytokine IL-16 can contribute to innate immunity by chemoattracting CD4-expressing peripheral immune cells along an IL-16 gradient for their recruitment and activation at sites of inflammation. The protean functionality of CD4 extends past immunity as CD4 also notably serves as the major receptor for HIV-1 and human herpes virus 7 (HHV-7) infections. During HIV pathogenesis, CD4 acts instrumentally as a high-affinity entry receptor for the internalization of HIV-1 following binding of the viral envelope glycoprotein gp120 to CD4ís extracellular domain.
CD14 is a cell surface-anchored glycoprotein that is expressed predominantly by monocytes and tissue macrophages. CD14 associates with MD-2 (LY-96) and TLR4 to form a receptor complex, which signals specifically in response to bacterial lipopolysaccharide (LPS) binding. The CD14/MD-2/TLR4 receptor complex signals via MyD88, TIRAP, and TRAF6, and ultimately activates NF-kB. CD14 also exists in a soluble form, designated as sCD14, which is capable of specifically binding LPS in the extracellular space.
CD22 is a B-lineage restricted 135 kDa glycoprotein whose cell surface expression is limited to resting and activated B lymphocytes. The physiological role of CD22 is still unknown. Targeted disruption of CD22 in mice results in a reduced level of surface IgM on peripheral B cells, suggesting a role for CD22 in limiting antigen receptor signaling. CD22 is a member of the Ig gene superfamily that uniquely binds a sialic acid-dependent ligand.
CD23, the low affinity receptor for IgE, belongs to the C-type lectin structural family and plays a role in the regulation of IgE synthesis and IgE mediated activities. It is found both as a transmembrane receptor protein and in a soluble form, which is generated by proteolytic cleavage of membrane-bound CD23. The predominant soluble form of CD23 (sCD23) consists of 172 amino acids corresponding to the extracellular domain of the full length precursor. sCD23, in addition to binding IgE, also exerts a number of IgE-independent activities, such as promoting the activation and differentiation of B-cells and stimulating the release of pro-inflammatory cytokines from monocytes.
CD27 Ligand, a type II transmembrane protein, is a member of the TNF superfamily. It is expressed on activated T and B lymphocytes, as well as NK cells. CD27L and its receptor (CD27) regulate the immune response by promoting T-cell expansion and differentiation, as well as NK enhancement. CD27 signaling can act as a co-stimulatory effector to sustain the survival of CD8+ T cells, primarily by inducing increased expression of the IL-2 gene. Full length human CD27L is a 193 amino acid protein, consisting of a 17 amino acid cytoplasmic domain, a 21 amino acid transmembrane domain, and a 155 amino acid extracellular domain. Human soluble CD27L corresponds to the 155 amino acid extracellular domain of the full length CD27L protein.
CTLA-4 and CD28 are receptors of the immunoglobulin superfamily that are expressed, along with the transmembrane glycoproteins B7-1 and B7-2, by antigen-presenting cells, and with these ligands constitute crucial co-stimulatory pathways for T and B cell regulatory responses. It is through engagement with CD28 and CTLA-4 that the B7 family ligands B7-1 and B7-2 play principal roles in immunity by activating immune response and maintaining immune tolerance. Co-stimulatory signals generated by B7-1 and B7-2 interactions with CD28 serve to stimulate T cell activation and prevent anergy through the amplification of T cell receptor (TCR) signaling. In contrast, interactions of the ligands with CTLA-4 serves to maintain T cell homeostasis and self-tolerance through the disruption of stimulatory signaling from B7 isoform-bound CD28 complexes, and by inducing powerful inhibitory signals in T cells. CTLA-4, like B7-1, is only poorly expressed on resting dendritic cells; therefore, up-regulation of their interaction and resultant amplification and regulation of T cell activity at peripheral inflammation sites is considerably delayed upon immune activation. Conversely, B7-2 and CD28 are constitutively expressed by resting hematopoietic and T cells, respectively, and as a result are able to rapidly induce up-regulation upon immune activation, making them critical to the early co-stimulatory signaling of immune response. Unlike B7-1 and B7-2, the ligands PD-L1 (or B7-H1) and B7-H2, which also belong to the B7 family, have not been shown to influence immunity through interaction with CTLA-4. B7-H2 has been shown to have restricted interaction with CD28. The difference in expression of B7-1, B7-2 and B7-H2 may enable temporally and spatially-specific regulation of T cell response through non-competitive CD28 interaction.
CD30 ligand (CD30L) is a type-II membrane-associated glycoprotein belonging to the TNF superfamily and is expressed primarily on certain B cells, T cells, and monocytes. CD30L binds specifically to CD30 (receptor), which is expressed on activated, but not resting, B and T cells, in lymphomas and various chronically inflamed tissues. CD30L/CD30 interactions initiate a signaling cascade that can ultimately lead to the activation of NF-kB. CD30L/CD30 signaling exerts pleiotropic effects on normal cells, including cell death, differentiation, and cell division. Certain diseases, including Hodgkinís lymphoma, allergic inflammation, diabetes (in NOD mice), and mycobacterial infection can also be affected by CD30L/CD30 signaling. The CD30L gene encodes for a 234 amino acid type II transmembrane protein, which contains a 37 amino acid cytoplasmic sequence, a 25 amino acid transmembrane domain and a 172 amino acid extracellular domain.
CD40, a member of the TNF receptor superfamily, is a cell surface protein expressed on B cells, dendritic cells, monocytes, thymic epithelial cells and, at low levels, on T cells. Signaling though CD40 plays an important role in the proliferation and differentiation of B cells, and is critical for immunoglobulin (Ig) class switching. The membrane-anchored CD40-Ligand is expressed almost exclusively on activated CD4+ T lymphocytes. Failure to express CD40L leads to "immunodeficiency with hyper-IgM", a disease characterized by failure to produce IgG, IgA and IgE. The human CD40L gene codes for a 261 amino acid type II transmembrane protein, which contains a 22 amino acid cytoplasmic domain, a 24 amino acid transmembrane domain, and a 215 amino acid extracellular domain. The soluble form of CD40L is an 18 kDa protein comprising the entire TNF homologous region of CD40L and is generatedsin vivosby an intracellular proteolytic processing of the full length CD40L.
The Semphorins are a large family of phylogenetically conserved proteins that play a pivotal role in maintaining homeostasis in the immune system. Twenty members of this family have been identified and categorized into eight subclasses based on sequence similarity and distinctive structural features. CD100, also known as Sema4D, is a 150 kDa transmembrane class IV semaphorin. Studies have shown that CD100 can induce monocyte migration, T-cell activation, and B-cell survival, as well as T/B cell and T/DC "cooperation". The CD100 precursor contains 862 amino acids, including a 21 a.a. signal sequence, a 713 a.a. extracellular domain, a 21 a.a. transmembrane sequence, and a 107 a.a. cytoplasmic region. The extracellular sequence contains several structural features, including a 479 a.a. "sema" domain, a 79 a.a. Ig-like sequence, and a 52 a.a. "Plexin-type repeat".
CDNF is a secreted neurotrophic factor that is expressed in brain, neuronal and certain non-neuronal tissues. It has been shown to promote survival, growth and function of dopamine-specific neurons. CDNF and its structural homolog, MANF, each contain an N-terminal saposin-like lipid binding domain, and a carboxyl-terminal domain, which is not homologous to previously characterized protein structures. CDNF and MANF can prevent 6-OHDA-induced degeneration of dopaminergic neurons by triggering survival pathways in a rat experimental model of Parkinsonís disease.
Chemerin is a secreted chemoattractant protein that can signal through the chemokine-like receptor-1 (CMKLR1). It is expressed in various tissues, including white adipose tissue, and circulates in blood as an inactive 143 amino acid precursor protein. Biologically active Chemerin is generated by proteolytic removal of C-terminal residues by several circulating proteases. Chemerin acts as a chemoattractant for cells expressing the CMKLR1 receptor, which includes certain dendritic cells, macrophages, and adipocytes.
CNTF is a potent neural factor that was originally characterized as a vital factor for the survival of chick ciliary neurons in vitro. CNTF is also important for the survival of other neural cell types, including primary sensory neurons, motor neurons, basal forebrain neurons and type 2 astrocytes. CNTF is highly conserved across species and exhibits cross-species bioactivity.
CTACK is a keratinocyte-derived CC chemokine which signals through the CCR10 receptor. Both CTACK and CCR10 are expressed in normal and irritated epithelial cells. CTACK selectively attracts CLA+ T-cells and directs them into the skin. CTACK contains the four highly conserved cysteine residues present in most CC chemokines. The mature protein contains 88 amino acid residues.
CTGF is a member of the CCN family of secreted cysteine-rich regulatory proteins, and is the major mitogenic and chemoattractant protein produced by umbilical vein and vascular endothelial cells. CTGF stimulates the proliferation and differentiation of chondrocytes, induces angiogenesis, promotes cell adhesion of fibroblasts, endothelial and epithelial cells, and binds to IGF, TGF b1 and BMP-4. Cell migration and adhesion are signaled through binding to specific cell surface integrins and to heparin sulfate proteoglycans CTGF (98 a.a.), a lower molecular weight isoform containing the C-terminal portion of the full length CTGF protein, exerts full heparin binding, cell adhesion, and mitogenic CTGF activity. Mature Human CTGF is a 38.0 kDa secreted protein containing 323 amino acid residues. CTGF is comprised of four distinct structural domains (modules), which are identified as IGF binding protein (IGFBP), von Willebrand Factor C (VWFC), thrombospondin type-I (TSP type-I), and C-terminal cysteine knot-like (CTCK) domains. Full length CTGF can be proteolytically cleaved in certain tissues to yield N-terminal truncated isoforms, which, depending on the isoform, contain only the TSP type-I and CTCK domains or contain only the CTCK domain.
CTGFL/WISP-2 is a 28.6 kDa protein that belongs to the CCN family of cysteine-rich regulatory proteins. Members of this family stimulate mitosis, adhesion, apoptosis, extracellular matrix production, growth arrest, and migration of multiple cell types. The protein is expressed in primary osteoblasts, fibroblasts, the ovaries, testes, and heart. In addition to promoting adhesion of osteoblasts, CTGFL/WISP-2 inhibits osteocalcin production, as well as binding of fibrinogen to integrin receptors. Mature human CTGFL/WISP-2 is a 24.8 kDa polypeptide protein containing 227 amino acids. It is composed of 3 distinct domains; the IGF-Binding Protein domain (IGF-BP), the Thrombospondin type I repeat (TSP type I), and von Willebrand Factor C motif (VWFC).
CTLA-4 and CD28 are receptors of the immunoglobulin superfamily that are expressed, along with the transmembrane glycoproteins B7-1 and B7-2, by antigen-presenting cells, and with these ligands constitute crucial co-stimulatory pathways for T and B cell regulatory responses. It is through engagement with CD28 and CTLA-4 that the B7 family ligands B7-1 and B7-2 play principal roles in immunity by activating immune response and maintaining immune tolerance. Co-stimulatory signals generated by B7-1 and B7-2 interactions with CD28 serve to stimulate T cell activation and prevent anergy through the amplification of T cell receptor (TCR) signaling. In contrast, interactions of the ligands with CTLA-4 serves to maintain T cell homeostasis and self-tolerance through the disruption of stimulatory signaling from B7 isoform-bound CD28 complexes, and by inducing powerful inhibitory signals in T cells. CTLA-4, like B7-1, is only poorly expressed on resting dendritic cells; therefore, up-regulation of their interaction and resultant amplification and regulation of T cell activity at peripheral inflammation sites is considerably delayed upon immune activation. Conversely, B7-2 and CD28 are constitutively expressed by resting hematopoietic and T cells, respectively, and as a result are able to rapidly induce up-regulation upon immune activation, making them critical to the early co-stimulatory signaling of immune response. Unlike B7-1 and B7-2, the ligands PD-L1 (or B7-H1) and B7-H2, which also belong to the B7 family, have not been shown to influence immunity through interaction with CTLA-4. B7-H2 has been shown to have restricted interaction with CD28. The difference in expression of B7-1, B7-2 and B7-H2 may enable temporally and spatially-specific regulation of T cell response through non-competitive CD28 interaction.
CXCL16 is a member of the CXC chemokine family and signals through the CXCR6 receptor. CXCL16 may play a role in attracting lymphocyte subsets during inflammation and may facilitate certain immune responses. The chemokine domain of CXCL16 contains six cysteine residues, including the four highly conserved cysteine residues characteristic of CXC chemokines. The CXCL16 gene codes for a 273 amino acid polypeptide, which includes a 29 amino acid cytoplasmic domain and transmembrane sequence containing approximately 20 amino acids. The extracellular portion of CXCL16 contains a chemokines domain and an extended C-terminal "mucin-like stalk" sequence. The extracellular domain contains 89 amino acid residues (86 a.a. residues for the murine homolog).
CYR61 is a member of the CCN family of secreted cysteine-rich regulatory proteins. CYR61 induces angiogenesis by stimulating the proliferation, migration, and adhesion of endothelial cells. Cell migration and adhesion are mediated through binding to specific cell surface integrins and to heparin sulfate proteoglycans. Increased expression of CYR61 is associated with several types of cancer, and correlates with the progression and estrogen independence of human breast cancers.
Human sDLL-4 comprises the extracellular signaling domain of DLL, a member of a structurally-related family of single-pass type I trans-membrane proteins that serve as ligands for Notch receptors. DLL-4 functions to specifically activate the Notch-1 and Notch-4 receptors. The Notch signaling pathway regulates endothelial cell differentiation, proliferation and apoptosis, and is essential for the development, maintenance and remodeling of the vascular system. Targeted deletion of the DLL-4 gene in mice resulted in severe vascular defects and death before birth. Up-regulation of DLL-4 expression has been implicated in the vascular development of certain tumors. The human DLL-4 gene consists of a 503 amino acid extracellular domain with one DSL domain, eight EGF-like repeats, a 21 a.a. transmembrane domain, and a 135 a.a. cytoplasmic domain.
EGF is a potent growth factor that stimulates the proliferation of various epidermal and epithelial cells. Additionally, EGF has been shown to inhibit gastric secretion, and to be involved in wound healing. EGF signals through a receptor known as c-erbB, which is a class I tyrosine kinase receptor. This receptor also binds with TGF-α and VGF (vaccinia virus growth factor).
EGF Receptor (EGFR, ErbB1) is a transmembrane protein that exerts tyrosine kinase activity upon ligand-induced activation. EGFR can be activated by binding EGF, or at least six other structurally related protein ligands, including TGFα, HB-EGF, Betacellulin (BTC), Amphiregulin, Epiregulin, and Epigen. Upon activation, EGFR initiates a signaling cascade, which includes dimerization and internalization, tyrosine phosphorylation, DNA synthesis of target genes and, ultimately, cell proliferation. EGFR signaling plays a role in the growth and differentiation of normal cells, but elevated EGFR activity is correlated with the development and pathogenesis of certain cancers.
EGF-L7 (Epidermal growth factor-like protein 7, Multiple EGF-like domains protein 7, VE-statin) is a multi-domain protein containing two EGF-like domains and one EMI domain. It is expressed almost exclusively in endothelial cells and functions to promote normal development of the vascular system, particularly tubulogenesis. EGF-L7 is capable of antagonistic binding to Notch receptors, resulting in the inhibition of Notch signaling in HUVEC and neural stem cells. In research models inducing hypoxia and subsequent reoxygenation (H/R), EGF-L7 can inhibit ICAM-1 expression and enhance the inhibition of NF-kB activation. Additionally, EGF-L7 can chemoattract endothelial cells and bind to the extracellular matrix. The overexpression of EGF-L7 is observed in various cancers, and is generally correlated with increased metastasis and a poor prognosis.
ENA-78 is a CXC chemokine that signals through the CXCR2 receptor. It is expressed in monocytes, platelets, endothelial cells, and mast cells. ENA-78 is a chemoattractant for neutrophils. Three N-terminal truncated variants of human ENA-78; ENA 5-78, ENA 8-78, ENA 9-78, contain 74, 71, and 70 amino acid residues, respectively, possess increased biological activity. ENA-78 contains the four conserved cysteine residues present in CXC chemokines, and also contains the ëELRí motif common to CXC chemokine that bind to the CXCR1 and CXCR2 receptors.
Endostatin is a naturally occurring 20 kDa polypeptide derived from the C-terminal portion of type XVIII collagen. It functions as an anti-angiogenic cytokine that is expressed in various organs with the highest levels in liver, lung and kidney. Endostatin inhibits angiogenesis by blocking the pro-angiogenic activities of VEGF and FGF-basic.
Proteases (also called Proteolytic Enzymes, Peptidases, or Proteinases) are enzymes that hydrolyze the amide bonds within proteins or peptides. Most proteases act in a specific manner, hydrolyzing bonds at, or adjacent to, specific residues, or a specific sequence of residues contained within the substrate protein or peptide. Proteases play an important role in most diseases and biological processes, including prenatal and postnatal development, reproduction, signal transduction, the immune response, various autoimmune and degenerative diseases, and cancer. They are also an important research tool, frequently used in the analysis and production of proteins. Enterokinase sequentially cleaves carboxyl side of D-D-D-D-K. Human Enterokinase is expressed as a linear 1019 amino acid polypeptide precursor glycoprotein. Proteolytic processing of this precursor generates the biologically active form of Enterokinase, which consists of two polypeptide chains (heavy chain and light chain) held together by a single disulfide bond, resulting in formation of a biologically active heterodimer. The heavy chain consists of 784 amino acid residues, and the light chain consists of 235 amino acid residues.
Eotaxin is a CC chemokine that signals through the CCR3 receptor. It is produced by IFN-g-stimulated endothelial cells and TNF-activated monocytes. Eotaxin selectively chemoattracts eosinophils and, along with Eotaxin-2 and Eotaxin-3, plays a key role in the regulation of eosinophil recruitment in the asthmatic lung and in allergic reactions.
Eotaxin-2 is a CC chemokine that signals through the CCR3 receptor. It is produced by activated monocytes and T lymphocytes. Eotaxin-2 selectively chemoattracts cells expressing CCR3, including eosinophils, basophils, Th2 T cells, mast cells, and certain subsets of dendritic cells. Additionally, Eotaxin-2 inhibits the proliferation of multipotential hematopoietic progenitor cells. The mature protein, which also includes a C-terminal truncation, contains 78 amino acid residues (92 a.a. residues for the murine homolog, without C-terminal truncation). Eotaxin-2 contains the four conserved cysteine residues present in CC chemokines.
Eotaxin-3 is a CC chemokine that signals through the CCR3 receptor. It is produced by endothelial cells stimulated with IL-4 or IL-13. Eotaxin-3 selectively targets cells expressing CCR3, including eosinophils, basophils, T cells and monocytes. Eotaxin-3 has similar activity to Eotaxin and Eotaxin-2, but the three Eotaxins share only a low degree of sequence homology.
Epigen is an EGF-related polypeptide growth factor that signals through the ErbB receptor-1. It is produced in several tissues, including the testis, liver, and heart, as well as in certain tumor cells. Epigen is mitogenic for fibroblasts and epithelial cells. Human Epigen is initially synthesized as a glycosylated 14.7 kDa transmembrane precursor protein, which is processed by proteolytic cleavage to produce a mature soluble sequence.
Epiregulin is an EGF-related growth factor that binds specifically to EGFR (ErbB1) and ErbB4, but not ErbB2 or ErbB3. It is expressed mainly in the placenta and peripheral blood leukocytes, as well as in certain carcinomas of the bladder, lung, kidney and colon. Epiregulin stimulates the proliferation of keratinocytes, hepatocytes, fibroblasts and vascular smooth muscle cells. It also inhibits the growth of several tumor-derived epithelial cell lines. Human Epiregulin is initially synthesized as a glycosylated 19.0 kDa transmembrane precursor protein, which is processed by proteolytic cleavage to produce a 6.0 kDa mature secreted sequence.
Erythropoietin (EPO) is a glycoprotein hormone that is principally known for its role in erythropoiesis, where it is responsible for stimulating proliferation and differentiation of erythroid progenitor cells. The differentiation of CFU-E (Colony Forming Unit-Erythroid) cells into erythrocytes can only be accomplished in the presence of EPO. Physiological levels of EPO in adult mammals are maintained primarily by the kidneys, whereas levels in fetal or neonatal mammals are maintained by the liver. EPO also can exert various non-hematopoietic activities, including vascularization and proliferation of smooth muscle, neural protection during hypoxia, and stimulation of certain B cells.
Selectins are a family of calcium-dependent type 1 transmembrane proteins. Endothelial (E)-selectin is a heavily glycosylated transmembrane protein expressed by activated endothelial cells in microvascular linings. E-selectin, along with P-selectin and L-selectin, initiate recruitment of circulating leukocytes from blood to sites of inflammation in the vascular lining through interaction with specific cell surface-associated carbohydrate determinants. E-selectin consists of an N-terminal type 1 lectin domain, an EGF-like domain, 6 sushi (CCP/SCR) domains, a transmembrane sequence, and a short cytoplasmic domain.
Exodus-2 is a CC chemokine that can signal through the CCR7 receptor. It is expressed in lymph nodes of certain endothelial cells, and in the spleen and appendix. Exodus-2 chemoattracts T and B lymphocytes and inhibits hematopoiesis. Exodus-2 contains six cysteine residues, including the four conserved cysteines present in CC chemokines.
Fas Ligand (FasL) is a member of the TNF superfamily that is expressed on the cell surface of activated T cells. Binding of FasL to Fas Receptor triggers apoptosis in Fas-bearing cells. FasL has the ability to kill T cells and activated B cells, which leads to down-regulation of the immune response. The mechanism of Fas-induced apoptosis involves recruitment of pro-caspase 8 through an adaptor molecule called FADD, followed by processing of the pro-enzyme into active forms. These active caspases then cleave various cellular substrates, leading to the eventual cell death. Both human and murine sFasL are fully active on human and murine cells.
Fas and Fas Ligand (FasL) belong to the TNF superfamily, and are type I and type II transmembrane proteins, respectively. Binding of FasL to Fas triggers apoptosis in Fas-bearing cells. The mechanism of apoptosis involves recruitment of pro-caspase 8 through an adaptor molecule called FADD, followed by processing of the pro-enzyme into active forms. These active caspases then cleave various cellular substrates, leading to the eventual cell death. sFasR is capable of inhibiting FasL-induced apoptosis by acting as a decoy receptor that serves as a sink for FasL. The full length Fas (receptor) is a 319 amino acid type I transmembrane protein, which contains a 157 amino acid extracellular domain, a 17 amino acid transmembrane domain, and a 145 amino acid cytoplasmic domain.
Fetuin A/AHSG is a human plasma glycoprotein belonging to the Cystatin family of protease inhibitors. It is highly expressed in embryonic cells and adult hepatocytes, and is expressed to a lesser extent in monocytes/macrophages. Fetuin A/AHSG is a major serum protein component that exerts various calcium-dependent physiological activities, and can mediate growth signaling in certain tumor cells. It also can act as a natural antagonist against specific TGF-β and BMP signaling proteins.
Proteins of the FGF superfamily of growth factors manifest only a modest degree of primary sequence homology, yet share the ability to signal through one or more of four tyrosine kinase receptors called FGFR1 through FGFR4. The FGFs play a central role during prenatal development, postnatal growth and regeneration of a variety of tissues, by promoting cellular proliferation and differentiation. All members of the FGF superfamily bind, with varying degrees of affinity to heparin sulfate proteoglycans, which serve as extracellular storage sites and in some cases appear to be involved in the activation of the FGF receptors.
FGF-acidic is one of 23 known members of the FGF family. Proteins of this family play a central role during prenatal development, postnatal growth and regeneration of a variety of tissues, by promoting cellular proliferation and differentiation. FGF-acidic is a non-glycosylated heparin binding growth factor that is expressed in the brain, kidney, retina, smooth muscle cells, bone matrix, osteoblasts, astrocytes and endothelial cells. FGF-acidic has the ability to signal through all the FGF receptors.
FGF-basic is one of 23 known members of the FGF family. Proteins of this family play a central role during prenatal development, postnatal growth and regeneration of a variety of tissues, by promoting cellular proliferation and differentiation. FGF-basic is a non-glycosylated, heparin-binding growth factor that is expressed in the brain, pituitary, kidney, retina, bone, testis, adrenal gland, liver, monocytes, epithelial cells and endothelial cells. FGF-basic signals through FGFR 1b, 1c, 2c, 3c and 4.
FGF-4 is a heparin-binding growth factor that is a member of the FGF family. Proteins of this family play a central role during prenatal development, postnatal growth and regeneration of a variety of tissues, by promoting cellular proliferation and differentiation. FGF-4 signals through the FGFR 1c, 2c, 3c, and 4.
FGF-5 is a secreted, heparin-binding growth factor that belongs to the FGF family. Proteins of this family play a central role during prenatal development, postnatal growth and regeneration of a variety of tissues, by promoting cellular proliferation and differentiation. FGF-5 binds to FGFR 1c and 2c, and plays a regulatory role in the hair growth cycle.
FGF-6 is a secreted, heparin-binding growth factor that is a member of the FGF family. Proteins of this family play a central role during prenatal development, postnatal growth and regeneration of a variety of tissues, by promoting cellular proliferation and differentiation. FGF-6 is expressed in leukemia cell lines with platelet megakaryocytic differentiation potential. It signals through FGFR 1c, 2c, and 4.
KGF (FGF-7) is one of 23 known members of the FGF family. Proteins of this family play a central role during prenatal development, postnatal growth, and the regeneration of a variety of tissues, by promoting cellular proliferation and differentiation. KGF (FGF-7) is a mitogen factor specific for epithelial cells and keratinocytes. KGF/FGF-7 signals through FGFR 2b. KGF (FGF-7) plays a role in kidney and lung development, as well as in angiogenesis and wound healing.
FGF-8 is a heparin-binding growth factor belonging to the FGF family. Proteins of this family play a central role during prenatal development, postnatal growth and regeneration of a variety of tissues, by promoting cellular proliferation and differentiation. There are 4 known alternate spliced forms of FGF8; FGF-8a, FGF-8b, FGF-8e and FGF-8f. The human and murine FGF-8a and b are identical, unlike human and mouse FGF-8e and f, which are 98% identical. FGF-8 targets mammary carcinoma cells and other cells expressing the FGF receptors.
FGF-9 is a heparin-binding growth factor that belongs to the FGF family. Proteins of this family play a central role during prenatal development, postnatal growth and regeneration of a variety of tissues, by promoting cellular proliferation and differentiation. FGF-9 targets glial cells, astrocytes cells and other cells that express the FGFR 1c, 2c, 3b, 3c, and 4.
FGF-10 is a heparin-binding growth factor that belongs to the FGF family. Proteins of this family play a central role during prenatal development, postnatal growth and regeneration of a variety of tissues, by promoting cellular proliferation and differentiation. FGF-10 is most related to KGF/FGF-7, and is expressed during the development and, preferentially, in adult lungs. It signals through the FGFR 2b.
FGF-16 is a heparin-binding growth factor that is a member of the FGF family. Proteins of this family play a central role during prenatal development, postnatal growth and regeneration of a variety of tissues, by promoting cellular proliferation and differentiation. FGF-16 signals through FGFR 2c and 3c. FGF-16 plays a role in the development of the central nervous system.
FGF-17 is a heparin-binding growth factor that is a member of the FGF family. Proteins of this family play a central role during prenatal development, postnatal growth and regeneration of a variety of tissues, by promoting cellular proliferation and differentiation. FGF-17 signals through the FGFR 1c, 2c, 3c, and 4. FGF-17 signals induction and patterning of embryonic brain.
FGF-18 is a heparin-binding growth factor that belongs to the FGF family. Proteins of this family play a central role during prenatal development, postnatal growth and regeneration of a variety of tissues, by promoting cellular proliferation and differentiation. FGF-18 is an essential regulator of long bone and calvarial development. FGF-18 signals through FGFR 1c, 2c, 3c, and 4.
The FGF family plays central roles during prenatal development and postnatal growth and regeneration of a variety of tissues, by promoting cellular proliferation and differentiation. FGF-19, a member of the FGF family, is a high-affinity heparin-dependent ligand for FGFR4. FGF-19 is expressed during brain development and embryogenesis.
FGF-20 is a secreted, heparin-binding growth factor that is a member of the FGF family. Proteins of this family play a central role during prenatal development, postnatal growth and regeneration of a variety of tissues, by promoting cellular proliferation and differentiation. FGF-20 signals through the FGFR 2c and 3c, and is expressed during limb and brain development.
FGF-21 is a secreted growth factor that is a member of the FGF family. Proteins of this family play a central role during prenatal development, postnatal growth and regeneration of a variety of tissues, by promoting cellular proliferation and differentiation. FGF-21, in the presence of b-Klotho as a protein cofactor, signals through the FGFR 1c and 4 receptors, and stimulates insulin-independent glucose uptake by adipocytes.
The FGF family plays a central role during prenatal development, postnatal growth and regeneration of a variety of tissues, by promoting cellular proliferation and differentiation. FGF-23, FGF-21 and FGF-19 constitute an atypical FGF subfamily whose ligands act as circulating hormones and require the participation of asKlothosprotein as a co-receptor for their signaling. FGF-23 is a bone-derived hormone that acts in the kidney to regulate phosphate homeostasis and vitamin D metabolism. The signaling receptor for FGF-23, a Klotho-FGFR1 (IIIc) complex, is an essential regulator of the renal sodium phosphate co-transporter and key vitamin D-metabolizing enzymes CYP27B1 and CYP24A1.
The FGF family plays a central role during prenatal development and postnatal growth, and the regeneration of a variety of tissues, by promoting cellular proliferation and differentiation. The FGF ligands bind to a family of type I transmembrane tyrosine kinase receptors, which leads to dimerization and activation by sequential autophosphorylation of specific tyrosine residues. Four genes encoding structurally related FGF receptors (FGFR-1 to -4) are known. Alternative splicing of the mRNAs generates numerous forms of FGFR-1 to -3. Alternate forms of FGF receptors can exhibit different specificities with respect to ligand binding. For example, the form designated as FGFR1a (IIc) interacts predominantly with FGF-acidic (FGF1) and FGF-basic (FGF2). A frequent splicing event involving FGFR-1 and -2 results in receptors containing all three Ig domains, referred to as the alpha isoform, or only IgII and IgIII, referred to as the beta isoform. Only the alpha isoform has been identified for FGFR-3 and FGFR-4. Additional splicing events for FGFR-1 to -3, involving the C-terminal half of the IgIII domain encoded by two mutually exclusive alternative exons, generate FGF receptors with alternative IgIII domains (IIIb and IIIc).
Flt3-Ligand is a growth factor that regulates proliferation of early hematopoietic cells. Flt3-Ligand binds to cells expressing the tyrosine kinase receptor Flt3. Flt3-Ligand, by itself does not stimulate proliferation of early hematopoietic cells, but synergizes with other CSFs and interleukins to induce growth and differentiation. Unlike SCF, Flt3-Ligand exerts no activity on mast cells. Multiple isoforms of Flt3-Ligand have been identified. The predominant biologically active form is anchored to the cell surface as the extracellular domain of a transmembrane protein (209 a.a.). The membrane-bound isoform can be proteolytically cleaved to generate a biologically active soluble isoform.
Follistatin is a secreted protein that binds to ligands of the TGF-b family and regulates their activity by inhibiting their access to signaling receptors. It was originally discovered as an activin antagonist whose activity suppresses expression and secretion of the pituitary hormone FSH (follicle stimulating hormone). In addition to being a natural antagonist, follistatin can inhibit the activity of other TGF-b ligands including BMP-2,-4,-6,-7, Myostatin, GDF-11, and TGF-b1. Follistatin is expressed in the pituitary, ovaries, decidual cells of the endometrium, and in some other tissues.
Fractalkine is a CX3CL chemokine that signals through the CX3CR1 receptor. Fractalkine has been shown to chemoattract monocytes, microglia cells and NK cells. Fractalkine is, at this time, the only CXC3C chemokine that contains three amino acid residues between the first and second cysteine residues of the chemokine domain. The Fractalkine gene encodes for a 397 amino acid precursor protein containing a 24 amino acid signal sequence, a chemokine domain, and a "mucin-like stalk" sequence, which is followed by the transmembrane domain containing approximately 20 amino acids, and a C-terminal cytoplasmic domain. The extracellular chemokine domain contains 76 amino acid residues, including the four conserved cysteine residues found in other chemokines.
Secreted Frizzled Related Proteins (sFRPs) modulate WNT signaling by binding directly to WNT proteins in a manner that affects their receptor binding and signaling capabilities. sFRP-1 is a widely distributed protein that can bind directly to WNT1, WNT2, and possibly other WNT proteins, and generally exerts anti-proliferative effects consistent with activity as a WNT antagonist. It also inhibits apoptosis, and has been found to be down-regulated in many solid tumors, but up-regulated in uterine leiomyomas.
Secreted Frizzled-Related Proteins (sFRPs) are a family of glycosylated Wnt antagonists characterized by a conserved cysteine-rich domain that shares homology with the cysteine-rich, extracellular domain Frizzled proteins use for the binding of Wnt proteins and receptors. Lacking the transmembrane and intracellular domains of the Frizzled proteins, sFRPs function as soluble modulators of the Wnt signaling pathway through the direct binding of Wnt proteins to this cysteine-rich domain, and the resultant inhibition of Wnt receptor binding and signaling capabilities. sFRP-4 is widely distributed in a variety of embryonic and adult tissues where it can function as a circulating antiangiogenic factor, a potent proapoptotic factor, an inhibitor of insulin secretion, and a suppressor of both tumor growth and metastatic potential through disruption of the Wnt signaling pathway. Research has demonstrated the existence of a direct correlation between the downregulation and/or absence of circulating sFRP-4 and the progression of several cancer types, including ovarian, endometrial, prostate and lung. Upregulation of circulating sFRP-4 has been linked to the deterioration of glucose metabolism in the case of type 2 diabetes, as well as the suppression of the keratinocyte hyperproliferation and epidermal hyperlasia that are definitive of psoriasis.
Follistatin-like protein 1 (FSTL1) is a widely-expressed, extracellular glycoprotein that is homologously grouped into the osteonectin (BM-40/SPARC) family of secreted proteins based on its possession of both a follistatin-like and extracellular calcium-binding domain. Initially identified as a TGF-b-inducible protein in a cloned mouse osteoblast cell line, FSTL1 has since been implicated in an array of cell-type-specific functions, such as the regulation of proliferation, differentiation, apoptosis and migration, as well as a number of biological processes, including embryonic development, inflammatory response, angiogenesis, tumorigenesis, and immune disease pathogenesis. Highly conserved across mammalian species and widely expressed in human tissues, FSTL1 can be upregulated through signaling mediators of the innate immune system, such as TLR4 agonists and the arthritogenic cytokine IL-1b via NFkB pathways, to stimulate the expression and secretion of pro-inflammatory cytokines, including TNF-a, IL-1b, IL-6 and IL-8. While cells of mesenchymal lineage are capable of FSTL1 production, FSTL1 expression is notably absent from cells of hematopoietic lineage under normal physiological conditions. Macrophages and monocytes are, however, capable of taking up FSTL1 at sites of inflammation where FSTL1 stimulation can cause the expression of caspase-1 and its resultant enzymatic cleavage of active IL-1b from pro-IL-1b. Whereas the overexpression of FSTL1 has been noted as a substantial contributor to the progression of immune diseases like rheumatoid arthritis (RA) and osteoarthritis (OA), diminished FSTL1 serum levels have been identified as playing a significant part in both ovarian and endometrial carcinogenesis, where it directly affects cell proliferation, migration and invasion.
Lectins, of either plant or animal origin, are carbohydrate-binding proteins that interact with glycoprotein and glycolipids on the surface of animal cells. The Galectins are lectins that recognize and interact with beta-galactoside moieties. Galectin-1 is an animal lectin that has been shown to interact with CD3, CD4, and CD45. It induces apoptosis of activated T-cells and T-leukemia cell lines, and inhibits the protein phosphatase activity of CD45.
Lectins, of either plant or animal origin, are carbohydrate-binding proteins that interact with glycoproteins and glycolipids on the surface of animal cells. The Galectins are lectins that recognize and interact with β-galactoside moieties. Galectin-3 regulates a number of biological processes, including embryogenesis, inflammatory responses, cell progression and metastasis. Galectin-3 is normally expressed in epithelia of a variety of tissues, including colon and endometrium, and in various inflammatory cells, including macrophages. Galectin-3 can function intracellularly, controlling the cell cycle and preventing T-cell apoptosis, and also extracellularly, by activating various cells, including monocytes/macrophages, mast cells, neutrophils, and lymphocytes. Expression of Galectin-3 is affected by neoplastic transformation, being up-regulated in certain types of lymphomas, and in thyroid and hepatic carcinomas. Conversely, it is down-regulated in other cancers such as colon, breast, ovarian, and uterine.
CXCL6, also known as LIX in mice, is a CXC chemokine that signals through the CXCR2 receptor. It is expressed in monocytes, platelets, endothelial cells, and mast cells. LIX is a chemoattractant for neutrophils. The two naturally occurring variants of LIX, LIX 1-78 (GCP-2) and LIX 9-78 (GCP-2), contain 78 and 70 amino acid residues, respectively. LIX contains the four conserved cysteine residues present in CXC chemokines, and also contains the ëELRí motif common to CXC chemokines that binds to the CXCR1 and CXCR2 receptors.
G-CSF is a hematopoietic growth factor that stimulates the development of committed progenitor cells to neutrophils and enhances the functional activities of the mature end-cell. It is produced in response to specific stimulation by a variety of cells, including macrophages, fibroblasts, endothelial cells and bone marrow stroma. G-CSF is being used clinically to facilitate hematopoietic recovery after bone marrow transplantation. Human and murine G-CSF is cross-species reactive.
GDF-2 belongs to the TGF-b cytokine family, whose members play an important role during prenatal development and postnatal growth, and the remodeling and maintenance of a variety of tissues and organs. GDF-2 is expressed mainly in non-parenchymal cells of the liver, but is also found in other various cells and tissues. GDF-2 can signal through the ALK1 receptor, and has been implicated in a number of physiologic events including the regulation of the hepatic reticuloendothelial system, glucose homeostasis, iron homeostasis, and the inhibition of angiogenesis.
GDF-3 is a member of the TGF-β superfamily of growth and differentiation factors, and is highly homologous to GDF-9. Unlike most TGF-β family members, GDF-3 and GDF-9 are not disulfide-linked dimers. GDF-3 is expressed in adult bone marrow, spleen, thymus, and adipose tissue. The expression of GDF-3 is upregulated in high-fat-fed wild-type FABP4/aP2 null mice and was associated with obesity, but not with the related hyperglycemia/hyperinsulinemia that characterizes Type 2 diabetes.
GDF-5 is expressed in long bones during embryonic development and postnatally in articular cartilage. Mutations in the GDF-5 gene have been implicated in Hunter-Thompson type dwarfism and in Grebe Syndrome, which is characterized by short stature, extra digits, and short and deformed extremities. The mature and functional form of GDF-5 is a homodimer of two 120 amino-acid polypeptide chain (monomers) linked by a single disulfide bond. Each GDF-5 monomer is expressed as the C-terminal part of a precursor polypeptide, which also contains a 27 amino acid signal peptide and a 354 amino acid propeptide. This precursor undergoes intracellular dimerization, and upon secretion it is processed by a furin-type protease.
GDF-7 belongs to the TGF-β superfamily of growth and differentiation factors. It is expressed selectively by roof plate cells that are located in the developing embryonic central nervous system, and has been shown to influence the neuronal identity of cells within the central nervous system. GDF-7 has also been implicated in the formation, maintenance, and repair of certain cartilage and ligament tissues.
GDF-11 is a myostatin-homologous protein that acts as an inhibitor of nerve tissue growth. GDF-11 has been shown to suppress neurogenesis through a myostatin-like pathway, which involves the arrest of the progenitor cell cycle in the G1 phase. Similarities between myostatin and GDF-11, which are 90% identical in their amino acid sequence, suggest that the regulatory mechanisms responsible for maintaining proper tissue size during neural and muscular development might be the same.
GDNF is a disulfide-linked, homodimeric neurotrophic factor structurally related to Artemin, Neurturin and Persephin. These proteins belong to the cysteine-knot superfamily of growth factors that assume stable dimeric protein structures. GDNF signals through a multicomponent receptor system, composed of a RET and one of the four GFRa (α1-α4) receptors. GDNF specifically promotes dopamine uptake and survival, and morphological differentiation of midbrain neurons. Using a Parkinsonís disease mouse model, GDNF has been shown to improve conditions such as bradykinesia, rigidity, and postural instability. The functional human GDNF ligand is a disulfide-linked homodimer consisting of two 15 kDa polypeptide chains called monomers. Each monomer contains seven conserved cysteine residues, including Cys-101, which is used for inter-chain disulfide bridging, and others that are involved in the intramolecular ring formation known as the cysteine knot configuration.
Proteases (also called Proteolytic Enzymes, Peptidases, or Proteinases) are enzymes that hydrolyze the amide bonds within proteins or peptides. Most proteases act in a specific manner, hydrolyzing bonds at, or adjacent to, specific residues, or a specific sequence of residues contained within the substrate protein or peptide. Proteases play an important role in most diseases and biological processes, including prenatal and postnatal development, reproduction, signal transduction, immune response, various autoimmune and degenerative diseases, and cancer. They are also an important research tool, as they are frequently used in the analysis and production of proteins. Glu-C cleaves at the Carboxyl side of E (can also cleave D under certain conditions).
GM-CSF is a hematopoietic growth factor that stimulates the development of neutrophils and macrophages, and promotes the proliferation and development of early erythroid megakaryocytic and eosinophilic progenitor cells. It is produced in endothelial cells, monocytes, fibroblasts and T lymphocytes. GM-CSF inhibits neutrophil migration and enhances the functional activity of the mature end-cells. The human and murine molecules are species-specific and exhibit no cross-species reactivity.
Granzyme B is a cysteine protease found in the cytoplasmic granules of cytolytic T lymphocytes (CTL) and natural killer (NK) cells. Granzyme B is required for the induction of target cell lysis, which occurs as part of cell-mediated immune responses, and can activate apoptosis in target cells by both caspase-dependent and caspase-independent mechanisms. Proteolytic cleavage of substrates by Granzyme B takes place primarily after aspartic acid residues.
Growth Hormone (GH), also known as somatotropin, is a pleiotropic cytokine of the hematopoietic growth factor superfamily, which encompasses most cytokines, hematopoietic growth factors, and related receptors, and includes the related growth hormone receptor, prolactin, placental lactogens, proliferins, and somatolactin (SST). GH is primarily recognized for its anabolic role in stimulating the growth and differentiation of muscle, bone, and cartilage. A number of other functions, including immunomodulatory actions, are also attributed to GH, due in part to the pervasive distribution of its receptors, and the indirect effects associated with GH-stimulated production of insulin-like growth factors (IGFs). Occurring predominantly in the somatotropes of the anterior pituitary, whereupon it is stored in secretory granules, production of GH has also been noted in many other tissues, including those of the hematopoietic system. The production and pulsatile release of circulating GH is very tightly regulated by both negative and positive feedback regulations of pituitary and hypothalamic hormones, such as Pituitary-specific Positive Transcription Factor 1 (POU1F1), Growth Hormone Releasing Hormone (GHRH), and somatostatin (SRIF). Deficient production of GH is associated with dwarfism and reduction of lean body mass, while overproduction is associated with acromegaly and gigantism, as well as breast tumor growth.
GRO-α/MGSA (CXCL1) / GRO-β (CXCL2) / GRO-γ (CXCL3)
All three isoforms of GRO are CXC chemokines that can signal through the CXCR1 or CXCR2 receptors. The GRO proteins chemoattract and activate neutrophils and basophils. GRO/MGSA also stimulates mitogenesis in certain human melanoma cells.
HB-EGF is an EGF-related growth factor that signals through the EGF receptor, and stimulates the proliferation of smooth muscle cells (SMC), fibroblasts, epithelial cells, and keratinocytes. HB-EGF is expressed in numerous cell types and tissues, including vascular endothelial cells, and vascular SMC, macrophages, skeletal muscle, keratinocytes, and certain tumor cells. The ability of HB-EGF to specifically bind heparin and heparin sulfate proteoglycans is distinct from other EGF-like molecules, and may be related to the enhanced mitogenic activity, relative to EGF, that HB-EGF exerts on smooth muscle cells. The human HB-EGF gene encodes a 208 amino acid transmembrane protein, which can be proteolytically cleaved to produce soluble HB-EGF.
HCC-1 is a CC chemokine that signals through the CCR1 receptor and chemoattracts blood monocytes. It is secreted by various tissues, including skeletal muscle, heart, spleen, liver, bone marrow and plasma. Mature HCC-1 is found in four different forms, which are distinguished by differential N-terminal truncation and contain 74, 72, 71, or 66 amino acid residues.
Neuregulin/Heregulin is a family of structurally related polypeptide growth factors derived from alternatively spliced genes (NRG1, NRG2, NRG3 and NRG4). To date, there are over 14 soluble and transmembrane proteins derived from the NRG1 gene. Proteolytic processing of the extracellular domain of the transmembrane NRG1 isoforms releases soluble growth factors. HRG1-β1 contains an Ig domain and an EGF-like domain; the latter is necessary for direct binding to receptor tyrosine kinases erb3 and erb4. This binding induces erb3 and erb4 heterodimerization with erb2, stimulating intrinsic kinase activity that leads to tyrosine phosphorylation. Although HRG1-β1's biological effects are still unclear, it has been found to promote motility and invasiveness of breast cancer cells, which may also involve up-regulation of expression and function of the autocrine motility-promoting factor (AMF).
HGF is a potent, mesenchymally-derived mitogen for mature parenchymal hepatocytes, and acts as a growth factor for a broad spectrum of tissues and cell types. HGF signals through a transmembrane tyrosine kinase receptor known as MET. Activities of HGF include the induction of cell proliferation, motility, morphogenesis, inhibition of cell growth, and enhancement of neuron survival. HGF is a crucial mitogen for liver regeneration processes, especially after partial hepatectomy and other liver injuries. Human and murine HGF are cross-reactive. Human HGF is expressed as a linear, polypeptide-precursor glycoprotein containing 697 amino acid residues. Proteolytic processing of this precursor generates the biologically active heterodimeric form of HGF, which consists of two polypeptide chains (α-chain and β-chain) held together by a single disulfide bond resulting in formation of a biologically active heterodimer. The α-chain consists of 463 amino acid residues and four kringle domains. The β-chain consists of 234 amino acid residues.
Histidine-proline-rich glycoprotein (HPRG), a member of the Cystatin structural superfamily, is an abundantly secreted multi-domain glycoprotein. Although the physiological function is largely unknown, HPRG potentially regulates physiological processes such as cell adhesion and migration, fibrinolysis, coagulation, complement activation, immune complex clearance and phagocytosis of apoptotic cells. HPRG can exert anti-angiogneic activity by stimulating apoptosis of endothelial cells.
HVEM belongs to the TNF Receptor superfamily of transmembrane proteins, and plays a role in the activation of T-cells and other lymphocytes. It is expressed in various cells and tissues, including spleen, thymus, lung, macrophages, and T-cells. HVEM activation induces a signaling cascade that results in the induction of transcription factors NF-kB and AP-1. LIGHT (TNFSF14) and TNF-β (TNFSF1) function as the ligands for HVEM, which can also bind specifically to herpes simplex virus glycoprotein D. Soluble HVEM can act as a "receptor decoy" resulting in inhibition of the activity of the HVEM ligands, LIGHT and TNF-b.
I-309 is a CC chemokine that signals through the CCR8 receptor. It is secreted by T lymphocytes, monocytes and mast cells. I-309 chemoattracts monocytes and Th2 differentiated T-cells, but not neutrophils. Human I-309 is active on murine cells. Unlike some CC chemokines, whose biologically active form in solution is a non-disulfide-linked dimer, the biologically active form of I-309 is monomeric.
ICAMs are members of the Ig superfamily of calcium-independent transmembrane glycoproteins. ICAM-1 is a ligand for the lymphocyte function-associated antigen (LFA) and Mac-1 integrins, as well as the major human rhinovirus receptor. The primary function of ICAM-1 is to provide adhesion between endothelial cells and leukocytes after stress or injury. The human ICAM-1 gene codes for a 505 amino acid transmembrane glycoprotein containing a 29 amino acid cytoplasmic domain, a 23 amino acid transmembrane domain, and a 453 amino acid extracellular domain.
Proteins of this family play an important role in inducing non-specific resistance against a broad range of viral infections. They also affect cell proliferation and modulate immune responses. Produced by peripheral blood leukocytes and lymphoblastoid cells, IFN-α is an acid-stable molecule that signals through IFN-α/βR, which is also used by IFN-b. Both IFNs have similar anti-viral activity and regulate expression of MHC class I antigens. IFN-α contains four highly conserved cysteine residues that form two disulfide bonds, one of which is necessary for biological activity.
IFN-γ is an acid-labile interferon produced by CD4 and CD8 T lymphocytes as well as activated NK cells. IFN-γ receptors are present in most immune cells, which respond to IFN-g signaling by increasing the surface expression of class I MHC proteins. This promotes the presentation of antigen to T-helper (CD4+) cells. IFN-g signaling in antigen-presenting cells, and antigen-recognizing B and T lymphocytes, regulates the antigen-specific phases of the immune response. Additionally, IFN-γ stimulates a number of lymphoid cell functions, including the anti-microbial and anti-tumor responses of macrophages, NK cells, and neutrophils. Human IFN-γ is species-specific and is biologically active only in human and primate cells.
IFN λ1, 2, and 3 (also known as IL-29, IL-28A and IL-28B respectively) are distantly related to the IL-10 family and interferons. All three IFN-λs use a distinct receptor system composed of an IFN- λR1 subunit (also called CRF2-12) and IL-10R2 subunit (also called CRF2-14). Signaling through this receptor system induces antiviral defenses similar to, but distinct from, that of type I interferons.
IFN-ω is a type I interferon that can be induced by virus-infected leukocytes. Members of the type I interferon family, which includes IFN-α, IFN-β, and IFN-ω, signal through the IFNAR-1/IFNAR-2 receptor complex, and exert antiviral and antiproliferative activities. IFN-ω exhibits about 75% sequence homology with IFN-α, and contains two conserved disulfide bonds that are necessary for full biological activity.
The IGFs are mitogenic, polypeptide growth factors that stimulate the proliferation and survival of various cell types, including muscle, bone, and cartilage tissues in vitro. IGFs are predominantly produced by the liver, although a variety of tissues produce the IGFs at distinctive times. The IGFs belong to the Insulin gene family, which also contains insulin and relaxin. The IGFs are similar to insulin by structure and function, but have a much higher growth-promoting activity than insulin. IGF-II expression is influenced by placenta lactogen, while IGF-I expression is regulated by growth hormone. Both IGF-I and IGF-II signal through the tyrosine kinase type I receptor (IGF-IR), but IGF-II can also signal through the IGF-II/Mannose-6-phosphate receptor. Mature IGFs are generated by proteolytic processing of inactive precursor proteins, which contain N-terminal and C-terminal propeptide regions. Recombinant human IGF-I and IGF-II are globular proteins containing 70 and 67 amino acids, respectively, and 3 intra-molecular disulfide bonds. IGF-I LR3 is a recombinant analog of human IGF-I comprised of the complete IGF-I sequence, with an Arginine substitution for the third position Glutamic acid, and a 13 amino acid length N terminus peptide extension. Specifically engineered for higher biological potencys in vitro, IGF-I LR3 has an increased half-life and a binding aversion to native proteins within the body that make it ideal for both research and large-scale culturing.
IGF-BPs control the distribution, function and activity of IGFs in various cell tissues and body fluids. IGF-BP4 is the major IGF-BP produced by osteoblasts, and is found in the epidermis, ovarian follicles, and other tissues. IGF-BP4 inhibits the activity of IGF-I and IGF-II by binding in a manner that results in the formation of complexes with reduced ability to signal through cell surface IGF receptors. IGF-BP4 can inhibit the growth of chick pelvis cartilage and HT29 colon adenocarcinoma cells by blocking the mitogenic actions of IGFs, and has also been shown to reduce colony formation by colorectal cancer cells via an IGF-independent pathway. The biological effects of IGF-BP4 can be regulated by Pregnancy Associated Plasma Protein A (PAPP-A), which reduces IGF-BP4/IGF binding affinity by proteolytically cleaving IGF-BP4. The modulation of IGF-BP4 activity by PAPP-A is an important component in the regulation of ovarian folliculogenesis and in the growth inhibition of responding ovarian cancer cells.
IL-1α is a non-secreted, proinflammatory cytokine produced in a variety of cells, including monocytes, tissue macrophages, keratinocytes, and other epithelial cells. Both IL-1α and IL-1β bind to the same receptor and have similar, if not identical, biological properties. These cytokines have a broad range of activities including the stimulation of thymocyte proliferation by inducing IL-2 release, B-cell maturation and proliferation, mitogenic FGF-like activity and the release of prostaglandin and collagenase from synovial cells. However, whereas IL-1β is a secreted cytokine, IL-1α is predominantly a cell-associated cytokine.
IL-1 Receptor Antagonist (IL-1RA)
Interleukin-1 receptor antagonist (IL-1RA) is a naturally-occurring, inflammatory-inhibitor protein. It inhibits the activity of IL-1α and IL-1β by competitively blocking their binding to type I and type II receptors. IL-1RA is produced by corneal epithelial cells, monocytes, neutrophils, macrophages, and fibroblasts. Therapeutically, IL-1RA may help in the treatment of sepsis, cachexia, rheumatoid arthritis, chronic myelogenous leukemia, asthma, psoriasis, and inflammatory bowel disease.
IL-2 is a powerful immunoregulatory lymphokine produced by T-cells in response to antigenic or mitogenic stimulation. IL-2/IL-2R signaling is required for T-cell proliferation and other fundamental functions that are essential for the immune response. IL-2 stimulates growth and differentiation of B-cells, NK cells, lymphokine-activated killer cells, monocytes, macrophages and oligodendrocytes.
The IL-2 receptor system consists of three non-covalently linked subunits termed IL-2Rα, IL-2Rβ, and IL-2Rγ. The IL-2Rα is a type I transmembrane protein consisting of a 219 amino acid extracellular domain, a 19 amino acid transmembrane domain and a 13 amino acid intracellular domain, which is not involved in the transduction of IL-2 signals. Proteolytic processing of IL-2Rα releases the entire extracellular domain of IL-2Rα, thereby generating a 219 amino acid soluble protein called soluble IL-2Rα (sIL-2Rα). The homodimeric form binds IL-2 (KD=10mM) and facilitates IL-2 signaling. The secreted sIL-2Rα is expressed on leukemia cells, lymphoma cells, and newly activated T and B cells, as well as on approximately 10% of NK cells.
IL-3 is a hematopoietic growth factor that promotes the survival, differentiation and proliferation of committed progenitor cells of the megakaryocyte, granulocyte-macrophage, erythroid, eosinophil, basophil and mast cell lineages. Produced by T cells, mast cells and eosinophils, IL-3 enhances thrombopoiesis, phagocytosis, and antibody-mediated cellular cytotoxicity. Its ability to activate monocytes suggests that IL-3 may have additional immunoregulatory roles. Many of the IL-3 activities depend upon co-stimulation with other cytokines. IL-3 is a species-specific, variably glycosylated cytokine.
IL-4 is a pleiotropic cytokine that regulates diverse T and B cell responses including cell proliferation, survival and gene expression. Produced by mast cells, T cells and bone marrow stromal cells, IL-4 regulates the differentiation of naive CD4+ T cells into helper Th2 cells, characterized by their cytokine-secretion profile that includes secretion of IL-4, IL-5, IL-6, IL-10, and IL-13, which favor a humoral immune response. Another dominant function of IL-4 is the regulation of immunoglobulin class switching to the IgG1 and IgE isotypes. Excessive IL-4 production by Th2 cells has been associated with elevated IgE production and allergy.
IL-4 can signal through type I and type II receptor complexes, which share a common gamma chain (γc). The type I receptor contains, in addition to the γc, an IL-4Rα subunit, whereas the type II receptor contains the IL-13Rα. The secreted extracellular domain of IL-4Ra, called sIL-4Rα, binds IL-4 and antagonizes its activity. It plays an important role in regulating the differentiation of naïve CD4+ T cells and class switching to IgG1 and IgE.
IL-5 is a hematopoietic growth factor that stimulates the proliferation and activation of eosinophils. Produced by mast cells, T cells, and eosinophils, IL-5 plays an important role in inducing cell-mediated immunity against parasitic infections and certain tumors. Elevated levels of IL-5 lead to Eosinophilia, which may result in the induction of asthma and other allergic diseases. Human and murine IL-5 are cross-species reactive.
IL-6 is a pleiotropic cytokine that plays an important role in host defense by regulating immune and inflammatory responses. Produced by T cells, monocytes, fibroblasts, endothelial cells and keratinocytes, IL-6 has diverse biological functions. It stimulates B cell differentiation and antibody production, synergizes with IL-3 in megakaryocyte development and platelet production, induces expression of hepatic acute-phase proteins, and regulates bone metabolism. IL-6 signals through the IL-6 receptor system that consists of two chains, IL-6Rα and gp130. Murine IL-6 is inactive on human cells, while both human and murine are equally active on murine cells.
IL-6 mediates its biological effects through the type I IL-6 receptor system that consists of two chains, IL-6Rα and gp130. While the IL-6Rα chain is the binding component specific to IL-6, the gp130 chain only transmits signals of IL-6 when bound to IL-6Rα. The gp130 can also transmit signals from LIF, OSM, CNTF, IL-11 and CT-1 in conjunction with other receptor subunits. The low-affinity binding site for IL-6 is composed of IL-6Rα alone. IL-6Rα is expressed in a wide range of cells, including T cells, fibroblasts and macrophages. Soluble IL-6Rα, which consists of only the extracellular domain of the IL-6Rα chain, acts as an agonist of IL-6 activity at low concentrations.
IL-7 is a hematopoietic growth factor that primarily affects early B and T cells. Produced by thymic stromal cells, spleen cells and keratinocytes, IL-7 can also co-stimulate the proliferation of mature T cells in combination with other factors, such as ConA and IL-2. Human and murine IL-7 are cross-species reactive.
IL-8 is a proinflammatory CXC chemokine that can signal through the CXCR1 and CXCR2 receptors. It is secreted by monocytes and endothelial cells. IL-8 chemoattracts and activates neutrophils.
IL-9 is an immunoregulatory cytokine produced by IL-2 activated Th2 lymphocytes. IL-9 enhances the proliferation of T lymphocytes, mast cells, erythroid precursor cells and megakaryoblastic leukemia cell lines. Over-expression of IL-9 has been implicated in the pathogenesis of anaplastic lymphoma and Hodgkinís disease. Whereas murine IL-9 can function on human cells, human IL-9 is inactive on mouse cells.
IL-10 is an immunosuppressive cytokine produced by a variety of mammalian cell types including macrophages, monocytes, T cells, B cells and keratinocytes. IL-10 inhibits the expression of proinflammatory cytokines such as IL-1 and TNF-α. Like IL-4, IL-10 enhances humoral immune responses and attenuates cell-mediated immune reactions. Human IL-10 is active on murine cells, but murine IL-10 is inactive on human cells.
IL-11 is a multifunctional cytokine produced by stromal cells, such as fibroblasts, epithelial cells and osteoclasts. It is expressed in a wide variety of tissues, including thymus, lung, bone, connective tissue and central nervous system. IL-11 plays an important regulatory role in hematopoiesis by stimulating growth of myeloid, erythroid and megakaryocyte progenitor cells. It also regulates bone metabolism, inhibits production of proinflammatory cytokines, and protects against gastromucosal injury.
IL-12 is a potent regulator of cell-mediated immune responses and it induces IFN-g production by NK and T cells. It is produced by activated monocytes/macrophage cells, B lymphocytes and connective tissue-type mast cells. Among its biological activities, IL-12 promotes the growth and activity of activated NK, CD4+ and CD8+ cells, and induces the development of IFN-γ-producing Th1 cells.
IL-13 is an immunoregulatory cytokine produced primarily by activated Th2 cells, and also by mast cells and NK cells. Targeted deletion of IL-13 in mice resulted in impaired Th2 cell development and indicated an important role for IL-13 in the expulsion of gastrointestinal parasites. IL-13 exerts anti-inflammatory effects on monocytes and macrophages and it inhibits the expression of inflammatory cytokines such as IL-1b, TNF-a, IL-6 and IL-8. IL-13 has also been shown to enhance B cell proliferation and to induce isotype switching, resulting in increased production of IgE. Blocking of IL-13 activity inhibits the pathophysiology of asthma. Human and murine IL-13 are cross-species reactive.
IL-15 is an immunomodulating cytokine that stimulates the proliferation of T lymphocytes and shares many biological properties with IL-2. IL-15 exerts its biological activities primarily on T cells. It is also essential in the development, survival and activation of NK cells. Increased expression of IL-15 has been linked to with rheumatoid arthritis, inflammatory bowel disease, and diseases affiliated with retroviruses HIV and HTLV-I. Human IL-15 is biologically active on mouse cells as measured by the dose-dependent stimulation of the proliferation of mouse CTLL cells.
IL-16 is a CD8+ T cell-derived cytokine that induces chemotaxis of CD4+ T cells, CD4+ monocytes, and eosinophils. Analysis by gel filtration suggests that, under physiological conditions, hIL-16 exists predominantly as a noncovalently linked multimer, but that some IL-16 may exist as a monomer. However, only the multimeric form appears to possess chemotactic activity, suggesting that receptor cross-linking may be required for activity. IL-16 also induces expression of IL-2 receptor (IL-2R) and MHC class II molecules on CD4 + T cells. Human and murine IL-16 show significant cross-species reactivity.
The originally described IL-17 protein, now known as IL-17A, is a homodimer of two 136 amino acid chains that are secreted by activated T-cells, which act on stromal cells to induce production of proinflammatory and hematopoietic bioactive molecules. Today, IL-17 represents a family of structurally-related cytokines that share a highly conserved C-terminal region, but differ from one another in their N-terminal regions and in their distinct biological roles. The six known members of this family, IL-17A through IL-17F, are secreted as homodimers. IL-17A exhibits cross-species bioactivity between human and murine cells.
IL-17B is a disulfide-linked homodimer of two 161 amino acid polypeptide chains. It belongs to the IL-17 family of structurally-related cytokines that share a highly conserved C-terminal region, but differ from one another in their N-terminal regions and in their distinct biological roles. The six known members of this family, IL-17A through IL-17F, are secreted as homodimers. IL-17B is expressed by T-cells, and has been shown to stimulate release of TNF-α and IL-1β from cells of the monocyte lineage.
IL-17D is a disulfide-linked homodimer of two 185 amino acid polypeptide chains. It belongs to the IL-17 family of structurally-related cytokines that share a highly conserved C-terminal region, but differ from one another in their N-terminal regions and in their distinct biological roles. The six known members of this family, IL-17A through IL-17F, are secreted as homodimers. IL-17D has the ability to stimulate the production of IL-6, IL-8 and GM-CSF, and inhibits hemopoiesis of myeloid progenitor cells in colony-forming assays.
IL-17E is a disulfide-linked homodimer of two 145 amino acid polypeptide chains. It belongs to the IL-17 family of structurally-related cytokines that share a highly conserved C-terminal region, but differ from one another in their N-terminal regions and in their distinct biological roles. The six known members of this family, IL-17A through IL-17F, are secreted as homodimers. IL-17E stimulates secretion of IL-8, and induces activation of the transcription factor NF-kB in cells that express the IL-17BR receptor.
IL-17F, a member of the IL-17 family of structurally related cytokines, has been shown to stimulate the proliferation and activation of T-cells and PBMCs. IL-17F also regulates cartilage matrix turnover and inhibits angiogenesis.
IL-19 belongs to the IL-10 family of regulatory cytokines, which includes IL-10, IL-19, IL-20, IL-22, IL-24 and IL-26. Members of this family share partial homology in their amino acid sequences, but they are dissimilar in their biological functions. Preliminary data suggests that IL-19 is a proinflammatory cytokine, because it up-regulates IL-6 and TNF-a, and induces apoptosis through TNF-α. IL-19 signals through the type I IL-20R. Human and murine IL-19 share 71% amino acid sequence identity.
IL-20 is a member of the IL-10 family of regulatory cytokines, which includes IL-10, IL-19, IL-20, IL-22, IL-24 and IL-26. Members of this family share partial homology in their amino acid sequences, but they are dissimilar in their biological functions. IL-20 is a hematopoietic growth factor capable of stimulating colony formation by CD34+ multipotential progenitors, but not by other progenitor cells. IL-20 signals through a receptor system composed of type I IL-20Rα and type II IL-20Rβ. Over-expression of IL-20 in keratinocytes expressing both receptor subunits has been implicated in the induction of inflammatory skin disease.
IL-21 is a pleiotropic cytokine produced by CD4+ T cells in response to antigenic stimulation. Its action generally enhances antigen-specific responses of immune cells. The biological effects of IL-21 include: inducing the differentiation of T-cell-stimulated B-cells into plasma cells and memory B-cells; the stimulation of IgG production in conjunction with IL-4; and the induction of apoptotic effects in naïve B-cells and stimulated B-cells in the absence of T-cell signaling. Additionally, IL-21 promotes the anti-tumor activity of CD8+ T-cells and NK cells. IL-21 exerts its effect through binding to a specific type I cytokine receptor, IL-21R, which also contains the γ chain (γc) found in other cytokine receptors, including IL-2, IL-4, IL-7, IL-9 and IL-15. The IL-21/IL-21R interaction triggers a cascade of events, which includes activation of the tyrosine kinases JAK1 and JAK3, followed by activation of the transcription factors STAT1 and STAT3.
IL-22 is a member of the IL-10 family of regulatory cytokines, which includes IL-10, IL-19, IL-20, IL-22, IL-24 and IL-26. Members of this family share partial homology in their amino acid sequences, but they are dissimilar in their biological functions. Produced by T lymphocytes, IL-22 inhibits IL-4 production by Th2 cells, and induces acute phase reactants in the liver and pancreas. IL-22 signals through a receptor system consisting of IL-10Rβ/CRF2-4 and IL-22R, both of which are members of the class II cytokine-receptor family.
IL-23 is a proinflammatory, heterodimeric protein composed of two subunits: a unique p19 subunit, and a p40 subunit that is shared with IL-12. IL-23 is secreted by activated dendritic cells and macrophages, and signals though a receptor comprised of IL-23R complexed with IL-12Rβ2. IL-23 has been shown to enhance proliferation of memory T cells. It also stimulates the production of IFN-γ in NK cells, induces IL-17 production, and drives Th17-mediated responses.
IL-24 is a secreted glycoprotein belonging to the IL-10 structural family of cytokines. It is produced by a variety of cell types, including B cells, CD4+ cells, NK cells, lymph node DCs, monocytes, and melanoma cells. IL-24 can signal through the IL20R1/IL20R2 and IL22R1/IL20R2 receptors to initiate a signaling cascade, which includes the induction of JAK1/STAT3 phosphorylation. IL-24 is, functionally, a pleiotropic protein, but is generally characterized as an anti-cancer cytokine that can selectively inhibit growth of a wide variety of human cancer cells through activities that include: the induction of differentiation and apoptosis; and the suppression of angiogenesis and cell proliferation.
As a member of the IL-12 family of heterodimeric cytokines that also includes IL-12, IL-23, and IL-35, IL-27 is formed by the association of an IL-27-p28 subunit (also known as IL-30) with the Epstein-Barr Virus (EBV)-induced Gene 3 (EBI3) subunit (also known as IL-27B). Expressed by antigen-presenting cells (APCs) in the early phases of antigen-mediated activation, IL-27 acts as a critical initiator of adaptive immune responses by promoting the rapid clonal expansion of naïve CD4+ T cells, IFN-γ production, and Th1 polarization. IL-27 elicits its effects through receptor complexes IL-27R (also known as TCCR/WSX-1) and gp130, a receptor shared by IL-6. Mainly expressed in monocytes, endothelial cells, and dendritic cells, IL-27 plays an important role alongside IL-6 in the regulation of inflammation and autoimmunity; directly antagonizing IL-6ís stimulation of CD4+ T cell proliferation and Th17 differentiation.
IL-31 is a T cell-derived cytokine that shares several structural and functional characteristics with IL-6, Oncostatin M, LIF, and Cardiotrophin-1. It signals through a receptor complex comprised of GPL (also known as gp130-like receptor or IL-31RA) and Oncostatin-M receptor (OSMR). GPL/OSMR signaling is a strong activator of STAT3 and STAT5, and can also activate STAT1, JAK1, and JAK2 signaling pathways. IL-31-regulated immune responses have been implicated in skin physiology and inflammatory skin diseases.
Human IL-33 is a proinflammatory protein that shares structural and functional characteristics with the IL-1 cytokine family. It binds and signals through the IL-1RL1/ST2 receptor, to activate NF-kB and MAP kinases. IL-33 induces production of TH2 cell related cytokines, including IL-4, IL-5 and IL-13, and exerts multiple inflammation related bioactivities.
IL-34 is a homodimeric cytokine that is expressed in a range of tissues that include spleen, heart, brain, liver, kidney, lung, ovary, thymus, testis, small intestine, prostate and colon. IL-34 is a ligand for colony-stimulating factor-1 receptor (CSF1R), which also binds to CSF-1. It specifically binds CD14+ monocytes, promotes survival and proliferation of human peripheral blood monocytes, and stimulates macrophage colony formation by human bone marrow cells. IL-34, like human CSF-1, stimulates phosphorylation of MAPK1/ERK2 and MAPK3/ERK1.
Interleukin-35 is a glycosylated, disulfide-linked, heterodimeric protein consisting of the p35 subunit from IL-12 (IL-12a) and the b subunit from IL-27 (EBI3). IL-35 can be expressed by regulatory T-cells (Tregs), macrophages, and certain trophoblast and dendritic cells. It is induced in response to inflammation, and generally acts as an inflammation suppressor. IL-35 suppresses inflammation by exerting multiple activities, including the induction of regulatory T-cells and the suppression of Th17 cells.
IL-36 Receptor Antagonist (IL-36RA)
The IL-1 family is comprised of 11 structurally related ligands, including the recently re-named IL-36RA (IL-1F5), IL-36α (IL-1F6), IL-36β (IL-1F8), and IL-36γ (IL-1F9). The interaction of IL-36 ligands with the IL-1Rrp2 receptor (IL-1R6) can induce various activities, including dendritic cell maturation and activation. IL-36RA can antagonize the NF-kB signaling induced by either IL-36α, β or γ by binding to the IL-1Rrp2 receptor in a manner that prevents the initiation of functional signaling.
The IL-1 family is comprised of 11 structurally related ligands, including recently re-named IL-36α (IL-1F6), β (IL-1F8) and γ (IL-1F9). IL-36β is highly expressed in psoriatic plaques, and at lower levels in various other tissues. IL-36b signals through the IL-1Rrp2 (IL-1R6) receptor, which is primarily expressed on certain dendritic cells. The interaction of the IL-1Rrp2 receptor with IL-36 ligands induces dendritic cell maturation and activation. IL-36β also functions as an agonist of NF-kB, and stimulates the production of pro-inflammatory proteins, including IL-6, IL-8, BD-2, and BD-3.
IL-36γ (IL-1F9)
The IL-1 family is comprised of 11 structurally related ligands, including recently re-named IL-36α (IL-1F6), β (IL-1F8) and γ (IL-1F9). IL-36γ is highly expressed in psoriatic plaques and in tissues containing epithelial cells. IL-36g signals through the IL-1Rrp2 (IL-1R6) receptor, which is primarily expressed on certain dendritic cells. The interaction of the IL-1Rrp2 receptor with IL-36 ligands induces dendritic cell maturation and activation. IL-36γ also functions as an agonist of NF-kB, and can stimulate the inflammatory response in bronchial epithelial cells.
The IL-1 family is comprised of 11 structurally related ligands, including recently re-named IL-37 (IL-1F7), which acts as a modulator of the immune response. Reduction of IL-37 synthesis in PBMCs leads to increased production of pro-inflammatory cytokines including IL-1 alpha, IL-1 beta, IL-6 and TNF- alpha. The role of IL-37 as an inhibitor of the innate inflammatory response is also corroborated by the observation that it is highly expressed in synovial tissue from patients with rheumatoid arthritis. Full length IL-37 resides primarily in the cytoplasm, but after activation through cleavage by CASP1, it can translocate to the nucleus where it exerts its activity by direct interaction with SMAD3.
IP-10 is a CXC chemokine that signals through the CXCR3 receptor. IP-10 selectively chemoattracts Th1 lymphocytes and monocytes, and inhibits cytokine-stimulated hematopoietic progenitor cell proliferation. Additionally, it is angiostatic and mitogenic for vascular smooth muscle cells.
I-TAC is a "non-ELR" CXC chemokine that is regulated by interferon and signals through the CXCR3 receptor. I-TAC is chemoattractant for IL-2-activated T cells, but does not affect freshly isolated un-stimulated T cells, neutrophils, or monocytes.
Proteases (also called Proteolytic Enzymes, Peptidases, or Proteinases) are enzymes that hydrolyze the amide bonds within proteins or peptides. Most proteases act in a specific manner, hydrolyzing bonds at, or adjacent to, specific residues, or a specific sequence of residues contained within the substrate protein or peptide. Proteases play an important role in most diseases and biological processes, including prenatal and postnatal development, reproduction, signal transduction, immune response, various autoimmune and degenerative diseases, and cancer. They are also an important research tool, as they are frequently used in the analysis and production of proteins. Kex-2 cleaves at the carboxyl end of the recognition sequences Arg-Arg/X and Lys-Arg/X.
Klotho is a glycosylated protein that plays an important role in the regulation of phosphate and calcium homeostasis. Human Klotho exists in both membrane bound and secreted forms, and is predominantly expressed in the kidney convoluted tubules, and, to a lesser extent, in the brain, reproductive organs, endocrine glands, urinary bladder, skeletal muscle, placenta, and colon. The full length transmembrane form has a large extracellular domain composed of two homologous subunits termed KL1 and KL2, which contain 516 and 439 amino acid residues, respectively. The predominant circulating form, which is derived from alternative RNA splicing, contains the KL1 subunit and constitutes the N-terminal sequence of transmembrane Klotho. A third Klotho protein of about 128 kDa has been identified in the blood and cerebrospinal fluid. This circulating protein arises from the action of an as yet unidentified protease, which cleaves transmembrane Klotho just above and/or within the plasma membrane. Klotho has been shown to play a key role in the signaling cascade of fibroblast growth factor-23 (FGF-23), a bone-derived hormone that acts in the kidney to inhibit phosphate reabsorption and vitamin D biosynthesis. Klotho promotes FGF-23 signaling through binding to FGFRI (IIIc) which converts this canonical FGF receptor into a specific receptor for FGF-23. In the absence of Klotho the function of FGF-23 is literally abolished.
LAG-1 is CC chemokine that signals through the CCR5 receptor. LAG-1 is identical to MIP-1β (ACT II isotype) except for two amino acid substitutions; arginine for histidine at position 22 and serine for glycine at position 47 of the mature protein. LAG-1 chemoattracts monocytes, and exhibits activity as an HIV-suppressive factor.
LD78β is a CC chemokine that is closely related to MIP-1α. It signals through the CCR5 receptor and the beta-chemokine receptor, D6. LD78β has been shown to exhibit potent activity in HIV suppression assays.
LEC is a CC chemokine that can signal through the CCR8 and CCR1 receptors. It is expressed in the liver, spleen, and thymus. LEC is chemotactic towards monocytes and lymphocytes, but not neutrophils.
Encoded by the ob (obese) gene, Leptin is an adipose-derived cytokine that suppresses appetite and increases thermogenesis. Leptin exerts its anorectic effect via signaling through a hypothalamic receptor termed OB-R. Leptin has been shown to reduce body weight, food consumption, and plasma glucose levels in various in vivo models.
Encoded for, along with leptin, by the obese (ob) gene, leptin receptor is a single-transmembrane-domain protein of the Type I, or Class I, Cytokine Receptor Family. The full length isoform, OB-Rb, is highly expressed in hypothalamic neurons, T- cells, and the vascular endometrium, and is thought to be the only isoform capable of transducing intracellular signals. Isoform OB-Ra, which is widely distributed at varying levels of expression, demonstrates weak signal activity and has been implicated in the active transport of leptin across the blood-brain barrier. Through ligand-binding with leptin receptor and the subsequent JAK2/STAT3 signaling cascade, the adipose-derived cytokine leptin functions to suppress appetite and increase thermogenesis. Leptin and leptin receptor have also, more recently, been implicated in the regulation or immune function, reproduction, glucose homeostasis, bone metabolism, wound healing, hematopoiesis, and angiogenesis. Mutations of thesobsgene, which can result in leptin resistance and the down-regulation of ligand and/or receptor expression, have been connected to obesity and hypothalamic pituitary function in various in vivo models, including human, mouse, and rat.
LIF is a pleiotrophic factor produced by multiple cell types, including T cells, myelomonocytic lineages, fibroblasts, liver, heart and melanoma. LIF promotes long-term maintenance of embryonic stem cells by suppressing spontaneous differentiation. Other activities include the stimulation of acute phase protein synthesis by hepatocytes, stimulation of differentiation of cholinergic nerves, and suppression of adipogenesis by inhibiting the lipoprotein lipase in adipocytes. While human LIF is active on mouse cells and is widely used in the maintenance of murine ESC to prevent spontaneous differentiation, mouse LIF is not active on human cells due to its inability to bind to the human LIF receptor.
LIGHT belongs to the TNF family of ligands, and can signal through the herpes virus entry mediator type A receptor (HVEM, TNFRSF14), LTbR, or bind to a decoy receptor, DcR3. It is expressed in splenocytes, activated PBL, CD8+ tumor infiltrating lymphocytes, granulocytes, and monocytes. LIGHT has the ability to active NF-kB, to co-stimulate the activation of lymphocytes and to induce apoptosis in certain human tumor cells.
Lungkine is a CXC chemokine that is expressed in lung epithelial cells and, to a lesser extent, in certain fetal tissues. No human homolog has been identified, and a specific cell surface receptor has not yet been found. Lungkine expression in lung tissue is elevated in response to inflammation, at which time it acts to specifically recruit neutrophils and direct them into the lung airway.
Lymphotactin is the only known member of the C chemokine family, and signals through the receptor XCR1, formally known as GPR5. The spleen shows the highest level of lymphotactin compared to peripheral leukocytes, lung, colon and small intestine. Lymphotactin is chemotactic towards lymphocytes, but not towards monocytes or neutrophils.
Maspin (mammary serine protease inhibitor) is a non-inhibitory serpin that is expressed predominantly in normal mammary epithelial cells, but at significantly reduced levels or absent in most breast carcinomas. It has the ability to block the growth, invasiveness, and metastatic potential of breast and lung tumors. This anti-tumor activity is achieved, in part, by the contribution of maspin to the inhibition of angiogenesis, and its ability to preferentially promote apoptosis of tumor cells.
MCP-1 (CCL2) / MCP-2 (CCL8) / MCP-3 (CCL7) / MCP-4 (CCL13) / MCP-5 (CCL12)
The MCP proteins are members of the CC chemokine family that signal through CCR2 and, with the exception of MCP-1, other CCR receptors. The MCP proteins chemoattract and activate monocytes, activated T cells, basophils, NK cells, and immature dendritic cells. The MCP family cross-reacts across species.
M-CSF is a potent hematopoietic factor produced by a variety of cells, including lymphocytes, monocytes, fibroblasts, endothelial cells, myoblasts and osteoblasts. It is a key regulator of cellular proliferation, differentiation, and survival for blood monocytes, tissue macrophages, and their respective progenitor cells. M-CSF has been shown to play important roles in modulating dermal thickness and fertility. M-CSF is clinically used in the treatment of infection, malignancies and atherosclerosis. It facilitates hematopoietic recovery after bone marrow transplantation. Human M-CSF is reactive in murine systems, but the murine molecule exhibits no activity on human cells.
MDC is a CC chemokine that is produced in B cells, macrophages, monocyte-derived dendritic cells, activated NK cells, and CD4 T cells. It signals through the CCR4 receptor. MDC chemoattracts monocytes, dendritic cells and NK cells, and exerts HIV-suppressive activity. The 67 amino acid form of MDC displays reduced chemoattractant activity, but retains HIV-suppressive activity. Human MDC is cross-reactive on murine lymphocytes.
MEC is a secreted CC chemokine expressed primarily by epithelial cells of the bronchioles, salivary gland, mammary gland and colon. MEC signals through the CCR10 receptor, and chemoattracts resting CD4, CD8 T-cells and eosinophils. MEC contains six cysteines, including the four highly conserved cysteine residues present in CC chemokines.
MIA is the first discovered member of a family of secreted cytokines termed the MIA/OTOR family. The four known members of this family, MIA, MIA-2, OTOR and TANGO, each contain a Src homology-3 (SH3)-like domain. MIA is an autocrine growth regulatory protein, secreted from chondrocytes and malignant melanoma cells that promotes melanoma metastasis by binding competitively to fibronectin and laminin in a manner that results in melanoma cell detachment from the extracellular matrix in vivo. Elevated levels of MIA may represent a clinically useful marker for diagnosis of melanoma metastasis, as well as a potential marker for rheumatoid arthritis.
MIA-2 is a secreted cytokine, and a member of the MIA/OTOR family. Members of this family, which also includes MIA, OTOR, and TANGO, share a Src homology-3 (SH3)-like domain. MIA-2 is predominantly expressed in hepatocytes. Elevated levels of MIA-2 may represent a clinically useful marker for diagnosis of hepatic disease activity and severity.
Macrophage migration inhibitory factor (MIF) is a small secreted protein that can act as a pleiotropic pro-inflammatory cytokine, as well as an enzyme. MIF pro-inflammatory activity can be initiated by signaling through CD74 and CD44, resulting in the secretion of TNF-a, IL-1, IL-6, IL-8, and various MMPs. The enzymatic activity of MIF is characterized by its ability to act as a tautomerase, capable of catalyzing the keto-to-enol isomerization of keto-phenylpyruvate and L-dopachrome. It appears as though MIF catalytic activity is dependent upon a trimeric configuration and a free N-terminal proline residue.
MIG, a CXC chemokine, is produced by IFN-g stimulated monocytes, macrophages and endothelial cells. It signals through the CXCR3 receptor. MIG selectively chemoattracts Th1 lymphocytes, and also exerts other activities, including inhibition of tumor growth, angiogenesis, and inhibition of colony formation of hematopoietic progenitors. Human MIG is active on murine cells.
Both MIP-1α and MIP-1β are structurally and functionally related CC chemokines. They participate in host response to invading bacterial, viral, parasite and fungal pathogens, by regulating the trafficking, and activation state, of selected subgroups of inflammatory cells (e.g. macrophages, lymphocytes and NK cells). While both MIP-1a and MIP-1b exert similar effects on monocytes, their effect on lymphocytes differ; with MIP-1a selectively attracting CD8+ lymphocytes, and MIP-1b selectively attracting CD4+ lymphocytes. Additionally, MIP-1α and MIP-1β have also been shown to be potent chemoattractants for B cells, eosinophils and dendritic cells. Both human and murine MIP-1α and MIP-1β are active on human and murine hematopoietic cells.
MIP-3 is a CC chemokine that signals through the CCR1 receptor. MIP-3 chemoattracts monocytes, resting T lymphocytes, and neutrophils, but does not chemoattract activated lymphocytes. Additionally, MIP-3 has been shown to inhibit colony formation of bone marrow myeloid immature progenitors.
MIP-3α is a CC chemokine, expressed in the liver, lymph nodes, appendix, PBL and lungs that can signal through the CCR6 receptor. MIP-3α is chemotactic towards lymphocytes and dendritic cells. Additionally, it promotes the adhesion of memory CD4+ T cells, and inhibits colony formation of bone marrow myeloid immature progenitors.
MIP-3β is a CC chemokine, expressed in the thymus, lymph nodes, and in activated bone marrow stromal cells that signals through the CCR7 receptor. MIP-3β is a chemoattractant for T and B lymphocytes, and myeloid progenitor cells. Human MIP-3β is active on murine cells.
MIP-4 is a CC chemokine that is expressed in lymph nodes, lungs, placenta and bone marrow. MIP-4ís primary receptor is unknown. MIP-4 chemoattracts lymphocytes, and has been shown to exert activity on both CD4+ and CD8+ T cells.
MIP-5 is a CC chemokine that is expressed in the heart, skeletal muscle and adrenal gland. MIP-5 primarily signals through the CCR1 receptor, but has also been found to bind to CCR3. MIP-5 is chemotactic towards T cells and monocytes.
Matrix metalloproteinases (MMPs) are a family of endoproteases that require zinc and calcium for expressing catalytic activity. These enzymes play a central role in the maintenance and remodeling of the extracellular matrix. Elevated expression of their activity, caused either by up-regulation of their expression or down-regulation of their cognate inhibitors, has been implicated in various degenerative disorders, including arthritis, cardiovascular disease, skeletal growth-plate disorders, and cancer metastasis.
MMP-1 is a secreted collagenase with specificity toward Type I, II, III, VII, and X collagens.
MMP-2 is a secreted collagenase with specificity toward Type IV, V, VII, and X collagens.
MMP-3 degrades fibronectin, laminin, collagens III, IV, and X, and cartilage proteoglycans.
Myostatin is a TGF-β family member that acts as an inhibitor of skeletal muscle growth. This muscle-specific cytokine interacts with Activin type I and type II receptors, and suppresses myoblast proliferation by arresting cell-cycle in the G1 phase. Suppression of myostatin activity facilitates muscle formation, and may be useful in reducing and/or preventing adiposity and type-2 diabetes. Myostatin activity can be blocked by the activin-binding protein follistatin, and by the propeptide of myostatin. The amino acid sequence of mature myostatin is extremely conserved across species, and is the same in murine, rat, chicken, turkey, porcine, and human. Myostatin is expressed as the C-terminal part of a precursor polypeptide, which also contains a short N-terminal signal sequence for secretion, and a propeptide of 243 amino acids. After dimerization of this precursor, the covalent bonds between the propeptide and the mature ligand are cleaved by furin-type proteases. However, the resulting two proteins remain associated through non-covalent interactions, and are secreted as a latent complex.
NAP-2 is a CXC chemokine that can signal through the CXCR1 and CXCR2 receptors. It is produced in leukocytes by enzymatic processing of a precursor called platelet basic protein (PBP). NAP-2 chemoattracts and activates neutrophils.
Neuropoietin is a newly identified member of the IL-6 cytokine family. Members of this family, including IL-6, IL-11, oncostatin M, leukemia inhibitory factor (LIF), cardiotrophin-1 (CT-1), cardiotrophin-like cytokine, and CNTF, display a four-helix bundle structure, and signal through gp130-containing receptor complexes. Neuropoietin, which is predominantly expressed in neuroepithelia during embryonic life, acts through a receptor complex formed of a CNTF receptor-a component, gp 130, and LIF receptor. Like CNTF, it promotes the survival of embryonic motor neurons, and could increase the proliferation of neural precursor cells in the presence of EGF and FGF-2. Interestingly, the human neuropoietin gene has evolved toward a pseudogene, suggesting that alternative signaling via CNTF is an effective compensatory pathway.
Neurturin is a disulfide-linked homodimer neurotrophic factor structurally related to GDNF, artemin, and persephin. These proteins belong to the cysteine-knot family of growth factors that assume stable dimeric structures. Neurturin signals through a multicomponent receptor system, composed of RET and one of four GFRa (α1-α4) receptors. Neurturin promotes the development and survival of sympathetic and sensory neurons by signaling through a receptor system composed of RET and GFRα2.
β-NGF is a neurotrophic factor structurally related to BDNF, NT-3 and NT-4. These proteins belong to the cysteine-knot family of growth factors that assume stable dimeric structures. β-NGF is a potent neurotrophic factor that signals through its receptor β-NGFR, and plays a crucial role in the development and preservation of the sensory and sympathetic nervous systems. β-NGF also acts as a growth and differentiation factor for B lymphocytes, and enhances B-cell survival.
NNT-1/BCSF-3 is a neurotrophic factor with B-cell-stimulating capabilities. Expressed in the lymph nodes and spleen, NNT-1/BCSF-3 activates glycoprotein 130 (gp130) and leukemia inhibitory factor receptor member b (LIFR-β) by binding and inducing the tyrosine phosphorylation of, these receptors. In vitro, it supports the survival of chick embryo motor and sympathetic neurons. In mice, NNT-1/BCSF-3 induces serum amyloid A, causes body weight loss, and B-cell hyperplasia associated with increases in serum IgG and IgM.
Noggin belongs to a group of diffusible proteins that bind to ligands of the TGF-b family, and regulate their activity by inhibiting their access to signaling receptors. The interplay between TGF-b ligands and their natural antagonists has major biological significance during development processes, in which cellular response can vary considerably depending upon the local concentration of the signaling molecule. Noggin was originally identified as a BMP-4 antagonist whose action was critical for proper formation of the head and other dorsal structures. Consequently, noggin has been shown to modulate the activities of other BMPs including BMP-2,-7,-13, and -14. Targeted deletion of Noggin in mice results in prenatal death, and a recessive phenotype displaying a severely malformed skeletal system. Conversely, transgenic mice over-expressing noggin in mature osteoblasts display impaired osteoblastic differentiation, reduced bone formation, and severe osteoporosis.
NOV is a member of the CCN family of secreted, cysteine-rich regulatory proteins. The full length NOV protein contains four structural domains that confer distinct, and sometimes opposing, biological activities. Elevated expression of NOV is associated with certain tumors, including Wilmís tumor and most nephroblastomas. However, in other tumor types and certain cancer cell lines, increased tumorgenicity and proliferation is correlated with decreased NOV expression. Additionally, NOV induces cell adhesion and cell migration by signaling through specific cell surface integrins, and by binding to heparin sulfate proteoglycans and to fibulin 1C. NOV has also been reported to exert proangiogenic activities.
Defensins (alpha and beta) are cationic peptides with a broad spectrum of antimicrobial activity that comprise an important arm of the innate immune system. The a-defensins, which include NP-1, NP-2 and NP-3, are distinguished from the b-defensins by the pairing of their three disulfide bonds. In addition to antimicrobial activity, NP-1 exhibits chemotactic activity on dendritic cells. NP-1 is expressed as the C-terminal portion of an inactive precursor protein, which also contains a 19 amino acid N-terminal signal sequence and a 45 amino acid polypeptide. NP-1 contains a six-cysteine motif that forms three intra-molecular disulfide bonds.
Oncostatin M (OSM) is a growth and differentiation factor that participates in the regulation of neurogenesis, osteogenesis and hematopoiesis. Produced by activated T cells, monocytes and Kaposiís sarcoma cells, OSM can exert both stimulatory and inhibitory effects on cell proliferation. It stimulates the proliferation of fibroblasts, smooth muscle cells and Kaposiís sarcoma cells, but inhibits the growth of some normal and tumor cell lines. It also promotes cytokine release (e.g. IL-6, GM-CSF and G-CSF) from endothelial cells, and enhances the expression of low-density lipoprotein receptors in hepatoma cells. OSM shares several structural and functional characteristics with LIF, IL-6, and CNTF.
Osteoprotegerin (OPG) is a member of the TNFR superfamily that can act as a decoy receptor for RANKL. Binding of soluble OPG to sRANKL inhibits osteoclastogenesis by interrupting the signaling between stromal cells and osteoclastic progenitor cells, thereby leading to excess accumulation of bone and cartilage. OPG is expressed in a wide variety of tissues, including the adult heart, lung, kidney, liver, spleen, prostate, lymph node, and bone marrow. OPG is secreted both as a monomeric and a dimeric protein. Its primary structure consists of seven distinct domains, four of which correspond to the extracellular cysteine-rich domains of TNFR proteins and constitute the soluble OPG.
OX40L, a member of the TNF superfamily of structurally related proteins, exists primarily as a type II membrane-bound, non-covalently linked, homotrimeric protein. It is expressed on antigen-presenting cells (APCs), such as dendritic cells and activated B-cells, as well as on various other cells such as vascular endothelial cells, mast cells, and natural killer cells. OX40L signals specifically through the OX40 receptor, which is expressed predominantly on CD4+ T cells, but also on certain activated CD8+ T cells. OX40/OX40L functions as a costimulatory signal, which is required for a productive interaction between antigen-presenting cells and their target T-cells. It enhances cell proliferation and survival, and increases expression of RANTES, IL-2, IL-3, and IFNg. OX40/OX40L signaling plays an important role in immuno-regulatory communication, enabling the immune system to distinguish between ìfriend vs. foeî during activation; a mechanism typically termed immuno-tolerance.
Platelet Activating Factor (PAF) is a biologically active phospholipid, which exerts primarily proinflammatory activities by specifically signaling through G-protein-coupled receptors on platelets, neutrophils, and monocytes. Platelet Activating Factor Acetylhydrolase (PAF-AH) is a secreted protein that mediates PAF activity by specifically catalyzing hydrolysis of the "sn2" ester bond, resulting in the conversion of PAF to the biologically inactive lyso-PAF. PAF-AH can also interact with LDL particles to induce the hydrolysis of LDL-associated, oxidized phospholipids, generating lysophosphatidylcholine (lyso-PC) and other lysophospholipids.
PDGFs are disulfide-linked dimers consisting of two 12.0-13.5 kDa polypeptide chains, designated PDGF-A and PDGF-B chains. The three naturally occurring PDGFs, PDGF-AA, PDGF-BB and PDGF-AB, are potent mitogens for a variety of cell types, including smooth muscle cells, connective tissue cells, bone and cartilage cells, and some blood cells. The PDGFs are stored in platelet α-granules, and are released upon platelet activation. The PDGFs are involved in a number of biological processes, including hyperplasia, chemotaxis, embryonic neuron development, and respiratory tubule epithelial cell development. Two distinct signaling receptors used by PDGFs have been identified and named PDGFR-α and PDGFR-β. PDGFR-α is high-affinity receptor for each of the three PDGF forms. On the other hand, PDGFR-β interacts with only PDGF-BB and PDGF-AB.
The platelet-derived growth factor (PDGF) family of heparin-binding growth factors consists of five known members, denoted PDGF-AA, PDGF-BB, PDGF-AB, PDGF-CC and PDGF-DD. The mature and active form of these proteins, an anti-parallel, disulfide-linked dimer of two 12-14 kDa, polypeptide chains, is obtained through proteolytic processing of biologically inactive precursor proteins, which contain an N-terminal CUB domain and a PDGF/VEGF homologous domain. The PDGFs interact with two related protein tyrosine kinase receptors, PDGFR-α and PDGFR-β, and are potent mitogens for a variety of cell types, including smooth muscle cells, connective tissue cells, bone and cartilage cells, and certain tumor cells. They play an important role in a number of biological processes, including hyperplasia, chemotaxis, embryonic neuron development, and respiratory tubulesí epithelial cell development. Mature PDGFs are stored in platelet a-granules, and are released upon platelet activation. PDGF-AA, -AB, -BB and -CC signal primarily through the PDGF-Rα receptor, whereas PDGF-DD interacts almost exclusively with the PDGF-Rβ receptor.
Programmed death-ligand 1 (PD-L1), or B7-H1, is a transmembrane, co-stimulatory ligand of programmed cell death protein 1 (PD-1) that, along with B7-1 and B7-2, belongs to the B7 family and immunoglobulin superfamily. Though more notably expressed on activated T-cells, B-cells, myeloid cells, and a subset of thymocytes, PD-L1 is also expressed constitutively by nonlymphoid, parenchymal organs, including the heart, placenta, skeletal muscle, and lung; with the marked exception of the small intestine. As a member of the B7 family, PD-L1 plays a principal role in immunity: suppressing immune response against autoantigens and tumors, maintaining T-cell homeostasis, maintaining peripheral immune tolerance, and regulating T-cell-mediated cytokine secretion. Unlike B7-1 and B7-2, PD-L1 has not been shown to influence immunity through interaction with CD28, CTL4A or ICOS, but rather through interaction with PD-1, a weak structural homolog of CTLA4 that belongs to the same superfamily. Involvement of PD-1 suggests an inhibitory function during T-cell activation; however, evidence has demonstrated PD-L1ís conflicting responsibility for both the stimulation and inhibition of T-cell-mediated cytokine synthesis. While T-cell co-stimulation with PD-L1 induces proliferation and the secretion of IL-10 and IFN-g, muscle cell expression of PD-L1 has been shown to inhibit function of CD4 and CD8 T-cells by down-regulating cytokine secretion and the expression of T-cell activation markers. Augmented expression of PD-L1 has been linked to the inhibition of antitumor immune response in cancer, and the up-regulation of IL-10 production in HIV-infection, resulting in increased susceptibility of antigen-specific T-cells to apoptosis.
PECAM is transmembrane glycoprotein that belongs to the Ig-related superfamily of adhesion molecules. It is highly expressed at endothelial cell junctions, and is also expressed in platelets and most leukocyte sub-types. The primary function of PECAM-1 is the mediation of leukocyte-endothelial cell adhesion and signal transduction. PECAM-1 has been implicated in the pathogenesis of various inflammation-related disorders, including thrombosis, multiple sclerosis (MS), and rheumatoid arthritis. The human PECAM-1 gene codes for a 738 amino acid transmembrane glycoprotein that contains a 118 amino acid cytoplasmic domain, a 19 amino acid transmembrane domain, and a 574 amino acid extracellular domain.
PEDF is a noninhibitory serpin with neurotrophic, anti-angiogenic, and anti-tumorigenic properties. It is a 50 kDa glycoprotein produced and secreted in many tissues throughout the body. A major component of the anti-angiogenic action of PEDF is the induction of apoptosis in proliferating endothelial cells. In addition, PEDF is able to inhibit the activity of angiogenic factors, such as VEGF and FGF-2. The neuroprotective effects of PEDF are achieved through suppression of neuronal apoptosis induced by peroxide, glutamate, or other neurotoxins. The recent identification of a lipase-linked cell membrane receptor for PEDF (PEDF-R) that binds to PEDF with high affinity (Notari, I. et al. J Biol Chem., Vol. 281, 38022-38037) should facilitate further elucidation of the underlying mechanisms of this pluripotent serpin. To date, PEDF-R is the only signaling receptor known to be used by a serpin family member. The unique range of PEDF activities implicate it as a potential therapeutic agent for the treatment of vasculature-related neurodegenerative diseases, such as age-related macular degeneration (AMD) and proliferative diabetic retinopathy (PDR). PEDF also has the potential to be useful in the treatment of various angiogenesis-related diseases including a number of cancers.
Persephin is a disulfide-linked, homodimeric, neurotrophic factor structurally related to GDNF, artemin, and neurturin. These proteins belong to the cysteine knot family of growth factors that assume stable dimeric structures. Persephin signals through a multicomponent receptor system, composed of RET and one of four GFR α (α1-α4) receptors. The GFRα4 was first identified in chicken, and was later shown to be the preferential binding subunit for persephin. Persephin promotes the survival of ventral midbrain dopaminergic neurons and motor neurons after sciatic nerve oxotomy, and, like GDNF, promotes ureteric bud branching. However, in contrast to GDNF and neurturin, persephin does not support the survival of peripheral neurons.
PF-4 is a CXC chemokine that is expressed in megakaryocytes and stored in the α-granules of platelets. PF-4 is chemotactic towards neutrophils and monocytes, and has been shown to inhibit angiogenesis.
PlGF-1 is an angiogenic factor that belongs to the cysteine-knot superfamily of growth factors. PlGF-1 is expressed in placental tissues, the colon, and mammary carcinomas. It signals through the VEGFR-1/FLT1 receptor, and stimulates endothelial cell proliferation and migration.
PlGF-2 is an angiogenic factor that belongs to the cysteine-knot superfamily of growth factors. PlGF-2 is expressed in umbilical vein endothelial cells and placenta. It signals through the VEGFR-1/FLT1 receptor, and stimulates endothelial cell proliferation and migration. PlGF-2 also signals through Neuropilin (NP-1), and can bind with high affinity to heparin.
PlGF-3 is an angiogenic factor that belongs to the cysteine-knot superfamily of growth factors. PlGF-3 is expressed exclusively in the placenta. It signals through the VEGFR-1/FLT1 receptor, and stimulates endothelial cell proliferation and migration. PlGF-3 lacks heparin binding affinity.
PTHrP is a polypeptide hormone produced by almost every tissue of the body. PTHrP is closely related to parathyroid hormone (PTH), which is secreted from the parathyroid gland, and plays a central role in regulating the extracellular concentrations of calcium and phosphorous.
RANKL and RANK are members of the TNF superfamily of ligands and receptors that play an important role in the regulation of specific immunity and bone turnover. RANK (receptor) was originally identified as a dendritic cell-membrane protein, which, by interacting with RANKL, augments the ability of dendritic cells. These dendritic cells then stimulate naïve T-cell proliferation in a mixed lymphocyte reaction, promote the survival of RANK + T-cells, and regulate T-cell-dependent immune response. RANKL, which is expressed in a variety of cells, including osteoblasts, fibroblasts, activated T-cells and bone marrow stromal cells, is also capable of interacting with a decoy receptor called OPG. Binding of soluble OPG to sRANKL inhibits osteoclastogenesis by interrupting the signaling between stromal cells and osteoclastic progenitor cells, thereby leading to excess accumulation of bone and cartilage. Human RANKL is reactive on murine cells. Impairments in RANK signaling have been implicated in the induction of expansile osteolysis and Pagetís disease of bone (PDB2).
RANTES is a CC chemokine that can signal through the CCR1, CCR3, CCR5 and US28 (cytomegalovirus receptor) receptors. It is a chemoattractant towards monocytes, memory T cells (CD4+/CD45RO), basophils, and eosinophils. RANTES also has the capability to inhibit certain strains of HIV-1, HIV-2 and simian immunodeficiency virus (SIV).
RELMα belongs to a unique family of tissue-specific cytokines termed FIZZ (found in inflammatory zone) and RELM. The four known members of this family, resistin, RELMα, RELMβ, and RELMγ, are 85-94 amino acid, secreted proteins sharing a conserved C-terminal domain, characterized by 10 cysteine residues with a unique spacing motif of C-X11-C-X8-C-X-C-X3-C-X10-C-X-C-X-C-X9-C-C. RELMα and resistin are secreted exclusively by adipocytes, while RELMβ is expressed in the epithelium of the colon and small bowel. The physiological role and molecular targets of RELMa are still unknown.
RELMβ (Resistin-like molecule b/FIZZ2) is a disulfide-linked, homodimeric protein expressed in the epithelium of the colon and small bowel. The biological functions of RELMβ, and its molecular targets, are not fully known, but it has been suggested that it plays a regulatory role during inflammation, and may also act to establish links among adipose tissue, the intestine and the liver. Interestingly, the molecular structure of RELMb is highly homologous to that of the adipose-derived cytokines, resistin and RELMα. These proteins share a highly conserved C-terminal domain, characterized by 10 cysteine residues with a unique spacing motif of C-X11-C-X8-C-X-C-X3-C-X10-C-X-C-X-C-X9-C-C.
RELMγ belongs to a unique family of tissue-specific cytokines termed FIZZ (found in inflammatory zone) and RELM. The other three known members of this family, resistin, RELMα, and RELMβ, are 85-94 amino acid, secreted proteins sharing a conserved C-terminal domain, characterized by 10 cysteine residues with a unique spacing motif of C-X11-C-X8-C-X-C-X3-C-X10-C-X-C-X-C-X9-C-C. RELMg is most closely related to RELMα, but is distinctly secreted in the bone marrow, spleen, lung, and peripheral blood granulocytes. The physiological role of RELMγ may include the promotion or regulation of promyelocytic differentiation, although the specific molecular targets of RELMγ have not been identified.
Resistin belongs to a family of tissue-specific cytokines termed FIZZ (found in inflammatory zones) and RELM. The four known members of this family, resistin, RELMα, RELMβ, and RELMγ, share a highly conserved C-terminal domain, characterized by 10 cysteine residues with a unique spacing motif of C-X11-C-X8-C-X-C-X3-C-X10-C-X-C-X-C-X9-C-C. Resistin is an adipose-derived cytokine (adipokine) whose physiological function and molecular targets are largely unknown. Studies have shown that resistin suppresses insulinís ability to stimulate glucose uptake, and postulated that resistin might be an important link between obesity and Type 2 diabetes. Other studies have indicated that resistin expression is severely suppressed in obesity, and that it may act as a feedback regulator of adipogenesis.
SCF is a hematopoietic growth factor that exerts its activity by signaling through the c-Kit receptor. SCF and c-Kit are essential for the survival, proliferation and differentiation of hematopoietic cells committed to the melanocyte and germ cell lineages. Human SCF manifests low activity on murine cells, while murine and rat SCF are fully active on human cells. The human SCF gene encodes for a 273 amino acid transmembrane protein, which contains a 25 amino acid N-terminal signal sequence, a 189 amino acid extracellular domain, a 23 amino acid transmembrane domain, and a 36 amino acid cytoplasmic domain. The secreted soluble form of SCF is generated by proteolytic processing of the membrane anchored precursor.
SDF-1α (CXCL12) / SDF-1β (CXCL12)
SDF-1α and β are stromal-derived, CXC chemokines that signal through the CXCR4 receptor. SDF-1α and β chemoattract B and T cells, and have been shown to induce migration of CD34+ stem cells. Additionally, the SDF-1 proteins exert HIV-suppressive activity in cells expressing the CXCR4 receptor. Human and murine SDF-1 proteins act across species. SDF-1α and β contain the four highly conserved cysteine residues present in CXC chemokines. The mature SDF-1α protein is the result of alternative splicing of the SDF-1 gene and contains 68 amino acid residues. The mature SDF-1βprotein, produced by an N-terminal truncation of two additional amino acids, after removal of the signal sequence, contains 72 amino acid residues.
Semaphorins are a large group of structurally-related, secreted, GPI-anchored, transmembrane, cell-signaling molecules. There are 8 major classifications of Semaphorins (the first seven ordered by number, 1-7, and the eighth designated V for virus), which are characterized by the existence of a conserved 500 amino acid SEMA domain at the amino terminus. Classes 3, 4, 6, and 7 are found in vertebrates only, whilst class 5 is found in both vertebrates and invertebrates. Each class is then divided into additional subgroups based on shared structural characteristics. Semaphorins primarily function as axon growth cone guidance factors during neuronal development. Semaphorin 3A acts as a chemo-repellent to axons, and an inhibitor of the growth of axons by signaling through receptors, Neuropilin-1 and Plexin-A.
Murine SF-20 is a bone marrow stroma-derived growth factor. SF20 is expressed in bone marrow, spleen stroma cells, resting mononuclear cells, resting CD8+ and CD19+ cells, and activated CD8+ T cells, and has been shown to bind to the surface of cells expressing the receptor TSA-1 (Thymic shared Ag-1). Among its biological activities, SF20 stimulates the proliferation of FDCP2 cells (a mouse factor-dependent hemopoietic cell line) and mouse lymphoid cells.
Slit2 is a member of the Slit family that signals through the Roundabout (Robo) receptor as a repellent for axon guidance and neuronal migration, and also acts as a chemoattractant to vascular endothelial cells and a chemotaxis inhibitor for leukocytes. Slit2 is expressed primarily in the fetal lung, kidney, and adult spinal cord, and to a lesser extent in the adult adrenal gland, thyroid and trachea. Slit2 is initially synthesized as a 1499 amino acid precursor, which is subsequently cleaved into N-terminal and C-terminal fragments, designated as Slit2-N and Slit2-C respectively. The neurodevelopment-related activities, as measured by the ability to repel olfactory bulb axons and to induce branching in dorsal root ganglia axons, are contained only in the N-terminal fragment.
Members of the Hedgehog (Hh) family are highly conserved proteins that are widely represented throughout the animal kingdom. The three known mammalian Hh proteins, Sonic (Shh), Desert (Dhh) and Indian (Ihh), are structurally related, and share a high degree of amino acid sequence identity (e.g. Shh and Ihh are 93% identical). The biologically active form of each Hh molecule is obtained by autocatalytic cleavage of their precursor proteins, and each corresponds to approximately one half of the N-terminal portion of the precursor molecule. Although Hh proteins have unique expression patterns and distinct biological roles within their respective regions of secretion, they use the same signaling pathway and can be substituted for one another in experimental systems.
Sox2 belongs to a diverse family of structurally-related transcription factors whose primary structure contains a 79-residue DNA-binding domain, called high mobility group (HMG) box. It plays an essential role in maintaining the pluripotency of embryonic stem cells (ESC) and the determination of cell fate. Microarray analysis showed that Sox2 regulates the expression of multiple genes involved in embryonic development, including FGF-4, YES1 and ZFP206. Sox2 acts as a transcriptional activator after forming a ternary complex with Oct3/4 and a conserved non-coding DNA sequence (CNS1) located approximately 2 kb upstream of the RAX promoter. The introduction of Sox2, Oct4, Myc, and Klf4 into human dermal fibroblasts isolated from a skin biopsy of a healthy research fellow was sufficient to confer a pluripotent state upon the fibroblast genome. The reprogrammed cells thus obtained resemble ESC in morphology, gene expression, and in their capacity to form teratomas in immune-deficient mice. Sox2 and other transcription factors have been introduced into cells by DNA transfection, viral infection, or microinjection. Protein transduction using TAT fusion proteins represents an alternative methodology for introducing transcription factors and other nuclear proteins into primary, as well as transformed, cells.
SPARC/Osteonectin is a secreted, evolutionarily-conserved, collagen-binding glycoprotein that is involved in a variety of cellular activities. It is highly expressed in tissues undergoing morphogenesis, remodeling and wound repair. SPARC/Osteonectin and its related peptides bind to numerous proteins of the extracellular matrix (ECM), affect ECM protein expression, influence cellular adhesion and migration, and modulate growth factor-induced cell proliferation and angiogenesis. SPARC/Osteonectin consists of three domains: an N-terminal acidic region that binds calcium ions with low affinity, a module containing two EF-hand motifs that bind calcium with high affinity, and a cysteine-rich follistatin-like domain.
TACI, a member of the TNF Receptor superfamily, is expressed in the small intestine, spleen, thymus, peripheral blood leukocytes, activated T cells, and resting B cells. TACI can bind to both APRIL and BAFF, stimulate the activation of transcription factors NF-kB and AP-1, and can mediate the calcineurin-dependent activation of NF-AT (nuclear-factor of activated T cells). TACI also plays a key role in the stimulation of B and T cell function. Soluble TACI inhibits APRIL-stimulated proliferation of primary B cells by blocking the binding of APRIL to the membrane-anchored TACI receptor.
TAFA proteins are a newly discovered family of proteins, which are distantly related to MIP-1α, a member of the CC-chemokine family. TAFA mRNAs are highly expressed in specific brain regions. The biological function of TAFA-2 is still unknown.
TARC, a CC chemokine, is predominantly produced by dendritic cells in the thymus, and signals through the CCR4 receptor. TARC is chemotactic towards T cells.
TECK is a CC chemokine, specifically expressed by thymic stromal cells, and signals through the CCR9 receptor. TECK is chemotactic towards activated macrophages, thymocytes and dendritic cells.
TGF-α is an EGF-related polypeptide growth factor that signals through the EGF receptor, and stimulates the proliferation of a wide range of epidermal and epithelial cells. It is produced by monocytes, keratinocytes, and various tumor cells. TGF-α induces anchorage-independent transformation in cultured cells. Human, murine and rat TGF-α are cross-species reactive.
The three mammalian isoforms of TGF-β, TGF-β1, β2, and β3, signal through the same receptor and elicit similar biological responses. They are multifunctional cytokines that regulate cell proliferation, growth, differentiation and motility, as well as synthesis and deposition of the extracellular matrix. They are involved in various physiological processes, including embryogenesis, tissue remodeling and wound healing. They are secreted predominantly as latent complexes, which are stored at the cell surface and in the extracellular matrix. The release of the biologically active TGF-β isoform from a latent complex involves proteolytic processing of the complex and /or induction of conformational changes by proteins such as thrombospondin-1.
TGF-β1 is the most abundant isoform secreted by almost every cell type. It was originally identified for its ability to induce phenotypic transformation of fibroblasts, and recently it has been implicated in the formation of skin tumors.
TGF-β2 has been shown to exert suppressive effects on IL-2-dependent T-cell growth, and may also have an autocrine function in enhancing tumor growth by suppressing immuno-surveillance of tumor development.
TGF-β3's physiological role of still unknown, but its expression pattern suggests a role in the regulation of certain development processes.
Thrombomodulin (TM, CD141, THBD) is an endothelial cell-expressed, transmembrane glycoprotein that can form a complex with the coagulation factor, thrombin. The thrombomodulin/thrombin complex converts protein C to its activated form, protein Ca, which in turn proteolytically cleaves and deactivates factor Va and factor VIIIa, two essential components of the coagulation mechanism. This inactivation reduces the generation of additional thrombin, and thereby effectively prevents continued coagulation. Reduced levels of thrombomodulin can correlate with the pathogenesis of certain cardiovascular diseases, such as atherosclerosis and thrombosis. However, the serum levels of the truncated circulating form of thrombomodulin are typically elevated during inflammation and in the presence of various inflammatory-related diseases. The thrombomodulin protein contains 575 amino acids, including an 18 a.a. signal sequence, a 497 a.a. extracellular domain, a 24 a.a. transmembrane sequence, and a 36 a.a. cytoplasmic region.
TIGAR is a p53-inducible enzyme that catalyzes the hydrolysis of fructose-2-6 bisphosphate (F-2-6-BP) to fructose-6-phosphate and inorganic phosphate. F-2-6-BP is a powerful activator of 6-phosphofructose-1 kinase, the rate limiting enzyme of glycolysis. By lowering the intracellular level of F-2-6-BP, TIGAR expression leads to increased glucose processing via the pentose phosphate pathway, the major cellular source for NADPH. NADPH plays a key role is maintaining the cellular redox state by regenerating reduced glutathione, which is critical for cellular protection against mitochondrial-derived reactive oxygen species (ROS). Consequently, TIGAR expression modulates p53-induced apoptosis in response to ROS-assocaited DNA damage. Since elevated levels of F-2-6-BP are required for cell growth and proliferation, p53-induces TIGAR expression prevents otugrowth of cells harboring damaged DNA. Protein transduction using TAT fusion proteins represents an alternative methodology for introducing transcription factors and other intracellular proteins into primary, as well as transformed, cells.
TIMP-1 and TIMP-2 are extracellular inhibitors of MMPs, including MMP-1, -2, -3, -7, -8, -9, -10, -11, -12, -13, and -16. They belong to the I35 (TIMP) family of irreversible protease inhibitors that function as key modulators of extracellular matrix degradation during tissue development and remodeling. TIMP-1 can also act through an MMP-independent mechanism to promote erythropoiesis by stimulating proliferation and differentiation of erythroid progenitors. TIMP-2 can also act through a MMP-independent mechanism inhibiting endothelial cell proliferation in vitro and demonstrates anti-angiogenic activities in vivo.
Tissue factor is a transmembrane glycoprotein of the cytokine receptor superfamily that acts as a receptor for coagulation factor VII (fVII) to trigger initiation of the coagulation cascade in response to vascular injury. Expression of tissue factor occurs constitutively within most extravascular and perivascular cells and at high levels within critical organs and tissue. Tissue factor is not normally expressed freely on the surface of circulating blood cells due to its pro-coagulant effect, but is instead stored on the surface of mononuclear and endothelial cells in microparticles that can shed into circulation in response to vascular injury, pro-inflammatory cytokines, or microbial ligands. Tissue factor can also be shed into circulation by cancer cells where its expression in a number of cancer types has been linked to tumor progression, metastatic potential, thrombosis, and angiogenesis. Expression of tissue factor has been shown to be inducible by select cytokines in a number of cell types, including IL-1β and TNF-α in vascular endothelial cells and macrophages, and TNF-α, IL-6, and FGF-Basic in monocytes, among others.
TL-1A belongs to the TNF superfamily of ligands. It is expressed predominantly in endothelial cells, and to a lesser extent in the placenta, lung, kidney, skeletal muscle, pancreas, small intestine and colon. TL-1A inhibits endothelial cell proliferation and angiogenesis, and has been shown to induce NF-kB activation, caspase activity, and apoptosis in responding cell lines. TL-1A interacts with TNFRSF25/DR3 receptor, but can also bind to a decoy receptor TNFRSF21/DR6.
TLR-3 is a single-pass type I receptor that binds to and signals the presence of microbial pathogens and double stranded RNA (dsRNA) viruses. Signaling through TLR-3 can promote the NF-kB pathway to initiate innate and adaptive immune responses to bacterial and viral infections, as well as the p53 pathway to trigger apoptosis in cells infected with dsRNA viruses. TLR-3 belongs to a family of structurally-related toll-like receptors (TLRs) containing an N-terminal domain rich in leucine repeats, and a C-terminal intracellular Toll/interleukin (IL)-1 (TIL) domain. TLR-3 is expressed primarily in dendritic cells of the placenta and pancreas where it can reside on both sides of the plasma membrane, and in the endosomal compartment of the cells.
TNF-α is a pleiotropic pro-inflammatory cytokine secreted by various cells, including adipocytes, activated monocytes, macrophages, B cells, T cells and fibroblasts. It belongs to the TNF family of ligands, and signals through two receptors, TNFR1 and TNFR2. TNF-α is cytotoxic to a wide variety of tumor cells, and is an essential factor in mediating the immune response against bacterial infections. TNF-α also plays a role in the induction of septic shock, autoimmune diseases, rheumatoid arthritis, inflammation, and diabetes. Human and murine TNF-α demonstrate significant cross-species reactivity. TNF-a exists in two forms; a type II transmembrane protein, and a mature soluble protein. The TNF-α transmembrane protein is proteolytically cleaved to yield a soluble, biologically active, 17 kDa TNF-α, which forms a non-covalently linked homotrimer in solution.
TNF-β is a potent mediator of inflammatory and immune responses. It belongs to the TNF family of ligands, and signals through TNFR1 and TNFR2. TNF-b is produced by activated T and B lymphocytes, and has similar activities to TNF-α. Like TNF-α, TNF-β is involved in the regulation of various biological processes, including cell proliferation, differentiation, apoptosis, lipid metabolism, coagulation, and neurotransmission. TNF-β is secreted as a soluble polypeptide, but can form heterotrimers with lymphotoxin-β, which effectively anchors the TNF-β to the cell surface. TNF-b is cytotoxic to a wide range of tumor cells.
TNFRI belongs to the TNFR superfamily of transmembrane proteins, and is expressed in most cell types. Binding of either TNF-α or TNF-β to TNFRI initiates a signal transduction pathway that results in the activation of the transduction factor NF-kB, whose target genes are involved in the regulation of inflammatory responses, and, in certain cells induce apoptosis. Soluble TNF Receptor I (sTNFRI) is capable of inhibiting TNF-α and TNF-β activities by acting as a decoy receptor that serves as a sink for the TNF ligands. The human TNFRI gene encodes for a 455 amino acid type I transmembrane protein, which contains a 21 amino acid signal sequence, a 190 amino acid extracellular domain, a 23 amino acid transmembrane domain, and a 221 amino acid cytoplasmic domain.
TNFRII is a member of the TNFR family of transmembrane proteins, and is expressed in immune cells and certain endothelial cells. It is a high affinity receptor for TNF-α, but manifests a lower affinity to TNF-β. Signaling through this receptor regulates various biological processes, including cell proliferation, differentiation, apoptosis, lipid metabolism, coagulation, and neurotransmission. Soluble TNFRII is capable of inhibiting TNF-α-induced activities by acting as a decoy receptor. The human TNFRII gene encodes for a 461 amino acid type I transmembrane protein, which contains a 22 amino acid signal sequence, a 235 amino acid extracellular domain, a 30 amino acid transmembrane domain, and a 174 amino acid cytoplasmic domain.
TPO is a lineage-specific growth factor produced in the liver, kidney and skeletal muscle. It stimulates the proliferation and maturation of megakaryocytes, and promotes increased circulating levels of platelets in vivo. TPO signals through the c-mpl receptor, and acts as an important regulator of circulating platelets. Human and murine TPO exhibit cross-species reactivity. The human TPO gene encodes for a 353 amino acid glycoprotein, which contains a 21 amino acid signal sequence, a 15 amino acid erythropoietin-like domain, and a highly glycosylated 179 amino acid C-terminal domain.
TRAIL/Apo2L is a cytotoxic protein, which activates rapid apoptosis in tumor cells, but not in normal cells. TRAILinduced apoptosis is achieved through binding to two death-signaling receptors, DR4 and DR5. These receptors belong to the TNFR superfamily of transmembrane proteins, and contain a cytoplasmic ìdeath domain,î which activates the cellís apoptotic machinery.
sTRAIL Receptor-1 / sTRAIL Receptor-2
TRAIL Receptor-1/DR4 and TRAIL Receptor-2/DR5 belong to the TNFR superfamily of transmembrane proteins, and contain a cytoplasmic "death domain," which can activate the cellís apoptotic machinery. These receptors are activated by binding to either membrane-anchored or soluble TRAIL/Apo2L. The DR4 and DR5 receptors are both produced as type I transmembrane proteins, which contain an extracellular domain, a transmembrane domain, and a cytoplasmic domain. The recombinant soluble forms of DR4 and DR5 consist of the TNFR-homologous, cysteine-rich portion of their respective extracellular domains.
Triggering receptor expressed on myeloid cells-1 (TREM-1) is a type 1 transmembrane glycoprotein belonging to the immunoglobulin superfamily of cell surface receptors that is constitutively expressed on the surface of monocytes, neutrophils and macrophages. The first identified member of the TREM family of receptors, TREM-1 is a proponent of amplified inflammatory responses triggered by bacterial and fungal infection, and functions in association with the signal transduction adaptor molecule DAP12. TREM-1 activation induces the sustained secretion of proinflammatory chemokines (IL-8 and MCP-1) and cytokines (TNF-α, IL-β, and IL-6), the respiratory burst and degranulation of neutrophils, the upregulation of adhesion molecules involved in leukocyte extravasation, and tissue degradation. Involvement and increased expression of TREM-1, or soluble TREM-1, in infectious, as well as some non-infectious, inflammatory conditions may highlight the proteinís potential as a possible diagnostic marker in cases of severe inflammation, such as myocardial dysfunction related to severe sepsis and spontaneous preterm labor. TREM-1 involvement in inflammatory response has also been noted as a predictor of both progression and aggression in certain cancers
TSLP is a hemopoietic protein that is expressed in the heart, liver and prostate. TSLP overlaps biological activities with IL-7, and binds with the heterodimeric receptor complex consisting of the IL-7R α-chain (IL-7Rα) and the TSLP-specific chain (TSLPR). Like IL-7, TSLP induces phosphorylation of STAT3 and STAT5, but uses kinases other than the JAKs for activation. TSLP prohibits apoptosis and stimulates growth of the human acute myeloid leukemia (AML)-derived cell line MUTZ3. It induces the release of T cell-attracting chemokines TARC and MDC from monocytes, and activates CD11c (+) dendritic cells (DCs). TSLP-activated DCs have been shown to prime naÔve T cells to produce the proallergic cytokines (IL-4, IL-5, IL-13, TNF-α) while down-regulating IL-10 and IFN-g, suggesting a role in initiating allergic inflammation.
TWEAK belongs to the TNF family of ligands, and signals through TWEAKR, also known as TNFRSF12A. TWEAK is expressed in a variety of tissues, including the adult heart, pancreas, skeletal muscle, small intestine, spleen and peripheral blood lymphocytes. TWEAK has the ability to induce NF-kB activation and chemokine secretion, and to exert an apoptotic activity in certain cells, such as HT-29 human adenocarcinoma cells when cultured in the presence of IFN-γ. TWEAK also promotes proliferation and migration of endothelial cells. The human TWEAK gene encodes for a 249 amino acid type II transmembrane protein, which contains a 21 amino acid cytoplasmic domain, a 21 amino acid transmembrane domain, and a 207 amino acid extracellular domain.
TWEAKR belongs to the TNF family of transmembrane proteins, and contains a cytoplasmic "death domain," which can activate a cellís apoptotic machinery. It is expressed in the spleen, thymus, peripheral blood lymphocytes, colon, and small intestine. Signal transduction by TWEAKR can be activated by either membrane-anchored or soluble TWEAK.
Uteroglobin, which is a member of the Secretoglobin superfamily and is also known as Clara cell phospholipid-binding protein, is a multifunctional protein that can exert anti-inflammatory and anti-tumorigenic effects by binding small hydrophobic molecules such as phospholipids and prostaglandins. The small, non-glycosylated protein named for its high levels of expression in pre-implantation embryos, where it exhibits growth stimulatory effects, is produced and secreted by the non-ciliated, non-mucous Clara cells predominant in the epithelial surfaces of pulmonary airways, as well as other non-ciliated epithelia. Members of the Secretoglobin superfamily demonstrate a high level of structural conservation and are characterized as small, secretory homo- or heterodimers. In addition to sequestering pro-inflammatory mediators and carcinogens, Uteroglobin has been implicated in the inhibition of cell migration and invasion, platelet aggregation, and T cell differentiation.
VAP-1 is a type II membrane cell adhesion protein belonging to the copper/topaquinone oxidase family. It is primarily expressed on the high endothelial venules of peripheral lymph nodes and on hepatic endothelia. VAP-1 can catalyze the oxidative deamination of low molecular weight amines, and plays an important role in the migration of lymphocytes to inflamed tissue. Inhibition of VAP-1 can protect against inflammation-related damage to certain injured tissues. Additionally, VAP-1 can function as a significant prognostic marker for certain cancers and cardiovascular diseases.
Vaspin is a newly described adipocytokine expressed predominantly in visceral white adipose tissues. Structure analysis of vaspin predicts the presence of three β-sheets, nine α-helices, and one central loop, which are distinctive structural features of Serpin family members. The serpins are irreversible ("suicidal") serine-protease inhibitors, characterized by having more than 30% sequence homology with α1-antitrypsin and a conserved tertiary structure, which contains an exposed reactive center loop that acts as a pseudo-substrate for the target proteinase. Members of this family play an important role in a number of fundamental biological processes, including blood coagulation, fibrinolysis, complement activation, angiogenesis, inflammation, and tumor suppression. In humans, the serpins represent approximately 2% of total serum proteins, of which 70% is α1-antitrypsin. Vaspin exhibits 40.2% sequence identity with a1-antitrypsin. Yet, its protease inhibitory activity is still unknown. Vaspin mRNA expression in visceral fat is positively correlated with BMI and percent of body fat. Administration of vaspin to obese mice improved glucose tolerance and insulin sensitivity, reflected by normalized blood glucose levels. It also led to the reversal of altered expression of diabetes-relevant adipocytokines, including leptin, adiponectin, resistin, and TNF-α. These findings suggest a potential clinical use for Vaspin in ameliorating certain aberrations seen in the diabetic/obesity metabolic syndrome.
VCAM is a 110 kDa, cell surface integral membrane glycoprotein that belongs to the Ig-related superfamily of adhesion molecules. The primary function of VCAM-1 is the mediation of leukocyte-endothelial cell adhesion and signal transduction. VCAM-1 may play a vital role in the development of several diseases, including atherosclerosis and rheumatoid arthritis. The human VCAM-1 gene codes for a 715 amino acid transmembrane glycoprotein containing a 19 amino acid cytoplasmic domain, a 22 amino acid transmembrane domain, and a 674 amino acid extracellular domain.
VEGF is a potent growth and angiogenic cytokine. It stimulates proliferation and survival of endothelial cells, and promotes angiogenesis and vascular permeability. Expressed in vascularized tissues, VEGF plays a prominent role in normal and pathological angiogenesis. Substantial evidence implicates VEGF in the induction of tumor metastasis and intra-ocular neovascular syndromes. VEGF signals through three receptors; fms-like tyrosine kinase (flt-1), KDR gene product (the murine homolog of KDR is the flk-1 gene product) and the flt4 gene product.
VEGF-B, a member of the VEGF family, is a potent growth and angiogenic cytokine. It promotes DNA synthesis in endothelial cells, helps regulate angiogenesis and vascular permeability, and inhibits apoptosis in certain smooth muscle cells and neurons. VEGF-B is expressed in all tissues except the liver. It forms cell surface-associated, disulfide-linked homodimers, and can form heterodimers with VEGF-A. There are two known isoforms, formed by alternative splicing, which have been designated VEGF-B167 and VEGF-B186. Both forms have identical amino-terminal sequences encoding a cysteine knot-like structural motif, but differ in their carboxyl-terminal domains. Both VEGF-B isoforms signal only through the VEGFR1 receptor.
VEGF-C, a member of the VEGF/PDGF family of structurally related proteins, is a potent angiogenic cytokine. It promotes endothelial cell growth, promotes lymphangiogesis, and can also affect vascular permeability. VEGF-C is expressed in various tissues, but is not produced in peripheral blood lymphocytes. It forms cell surfaced-associated, non-covalent, disulfide-linked homodimers, and can bind and activate both VEGFR-2 (flk1) and VEGFR-3 (flt4) receptors. During embryogenesis, VEGF-C may play a role in the formation of the venous and lymphatic vascular systems. Both VEGF-C and VEGF-D are over-expressed in certain cancers, and the resulting elevated levels of VEGF-C or VEGF-D tend to correlate with increased lymphatic metastasis.
VEGF-D, a member of the VEGF/PDGF family of structurally related proteins, is a potent angiogenic cytokine. It promotes endothelial cell growth, promotes lymphangiogesis, and can also affect vascular permeability. VEGF-D is highly expressed in the lung, heart, small intestine and fetal lung, and at lower levels in the skeletal muscle, colon, and pancreas. It forms cell surface-associated, non-covalent, disulfide-linked homodimers, and can bind and activate both VEGFR-2 (flk1) and VEGFR-3 (flt4) receptors. During embryogenesis, VEGF-D may play a role in the formation of the venous and lymphatic vascular systems. It also participates in the growth and maintenance of differentiated lymphatic endothelium in adults. Both VEGF-C and VEGF-D are over-expressed in certain cancers, and the resulting elevated levels of VEGF-C or VEGF-D tend to correlate with increased lymphatic metastasis.
Vitronectin is a secreted glycoprotein that is synthesized in the liver. It circulates primarily in monomeric form, but can undergo conformational change to a structure that forms disulfide-linked multimers. The multimeric vitronectin can efficiently bind to, and incorporate into, the extracellular matrix. Within the matrix, vitronectin can support cell adhesion through binding to various integrins and other proteoglycans. Additionally, recombinant vitronectin can function as a chemically-defined matrix component in human embryonic stem cell renewal media.
WISP-1 is a member of the CCN family of secreted, cysteine-rich regulatory proteins. It is expressed in the heart, kidney, lung, pancreas, placenta, ovary, small intestine and spleen. WISP-1 is a β-catenin-regulated protein that can contribute to tumorigenesis, and has also been shown to play a role in bone development and fracture repair.
WISP-3 is a member of the CCN family of secreted cysteine rich regulatory proteins. It is predominantly expressed in the adult kidney, testis, and fetal kidney, but it is also found with weaker expression in the placenta, ovary, prostate, small intestine, and skeletally-derived cells. WISP-3 is required for normal postnatal skeletal growth and cartilage homeostasis.
Wnt-1 is a secreted protein that signals through the Frizzled family of cell surface receptors, and is required for normal embryonic development. Wnt-1 activation induces a complex signaling cascade that ultimately leads to the increased expression of over fifty genes. An important component of Wnt-1 signaling is the stabilization, and resulting accumulation, of the intracellular signaling protein, β-catenin. Wnt signaling induces and maintains the transformed phenotype, and, in certain embryonic cell lines, supports self-renewal in the absence of significant differentiation. Elevated levels of Wnt proteins are associated with tumorigenesis, and are present in numerous human breast cancers. Mature human Wnt-1 is a glycosylated protein containing 343 amino acid residues.
Wnt-3a belongs to the Wnt family of signaling proteins that play a key role in maintaining the integrity of embryonic and adult tissues. Expression of Wnt-3a occurs primarily along the dorsal midline across overlapping regions of the Central Nervous System (CNS). Wnt-3a signaling is essential for various morphogenetic events, including embryonic patterning, cell determination, cell proliferation, CNS development, and cytoskeletal formation. Like other members of this family, Wnt-3a contains a highly conserved lipid-modified, cysteine-rich domain that is essential for cell signaling. During a biochemical process called the canonical Wnt pathway, Wnt family members bind to and activate, seven-pass transmembrane receptors of the Frizzled family, ultimately leading to the disruption of b-catenin degradation. Intracellular accumulation of b-catenin increases translocation of the protein into the nucleus, where it binds to TCF/LEF transcription factors to promote gene expression. Lack of Wnt signaling disrupts transcriptional activation of tumor suppressor genes, and has been shown to result in neoplastic transformation, oncogenesis, and human degenerative diseases.
Wnt-7a belongs to the Wnt family of signaling proteins that play a key role in maintaining the integrity of embryonic and adult tissues. It is expressed in placenta, kidney, testis, uterus, fetal lung, and fetal and adult brain. Most Wnt proteins can signal though a mechanism called the canonical Wnt pathway, in which Wnt proteins bind to and activate seven-pass transmembrane receptors of the Frizzled family, ultimately leading to the disruption of b-catenin degradation. Intracellular accumulation of β-catenin increases translocation of the protein into the nucleus, where it binds to TCF/LEF transcription factors to induce the expression of numerous genes. Increased Wnt/β-catenin signaling is associated with tumorigenesis in a diverse set of human cancers. However, Wnt-7a/Frizzled-9 signaling has been shown to act as a tumor suppressor in non-small cell lung cancers.